Abstract
Purpose
To compare two different types of drug-eluting microspheres with regard to impact on HRQoL after first TACE, tumour response, peri-procedural complications, adverse events and 1-year survival in patients suffering from unresectable hepatocellular carcinoma (HCC).
Methods
HRQoL was prospectively assessed with validated questionnaires (EORTC QLQ-C30 and -HCC18) before and 2 weeks after treatment with their first drug-eluting beads (DEB-)TACE with either acrylamido-polyvinylalcohol-AMPS hydrogel microspheres (groupDCB; 20 patients) or polyvinyl alcohol-co-acrylic acid microspheres (groupHS; 16 patients). Baseline characteristics, peri-procedural complications, treatment-related adverse events and 1-year survival were compared between both types of microspheres. Treatment response and objective response rates (ORR) were analysed using established tumour response criteria. Subgroup analysis for pooled groups with small (groupSMALL; 21 patients) versus large particles (groupLARGE; 15 patients) was performed.
Results
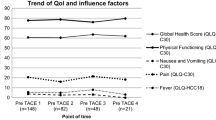
At baseline, there were no significant differences between the treated microsphere groups. No significant differences were found in absolute HRQoL changes after first DEB-TACE between the different types of microspheres. Response rates and survival were comparable between the investigated microsphere groups. For groupSMALL, we found a significant difference in post-interventional deterioration of physical function (− 19.4%) compared to groupLARGE (− 8%; p = 0.025). Tumour response and ORR according to mRECIST were significantly higher in groupSMALL (p = 0.008; p = 0.009).
Conclusion
DEB-TACE is generally well tolerated and effective, with comparable changes in HRQoL for both types of drug-eluting microspheres. Tumour response is better with small microspheres. A relevant deterioration of physical function underlines that an aggressive TACE using small beads should be well deliberated.



Similar content being viewed by others
Abbreviations
- AFP:
-
Alpha-fetoprotein
- AST:
-
Aspartate aminotransferase
- ALBI:
-
Albumin-Bilirubin
- ALT:
-
Alanine aminotransferase
- BCLC:
-
Barcelona Clinic Liver Cancer
- BMI:
-
Body mass index
- CPS:
-
Child–Pugh score
- CR:
-
Complete response
- CRP:
-
C-reactive protein
- DEB:
-
Drug-eluting bead
- EASL:
-
European Association for the Study of the Liver
- EORTC:
-
European Organisation for Research and Treatment of Cancer
- GHS:
-
Global health status
- HBV/HCV:
-
Hepatitis B/C virus
- HCC:
-
Hepatocellular carcinoma
- (HR)QoL:
-
(Health-related) quality of life
- MELD:
-
Model of End Stage Liver Disease
- mRECIST:
-
modified Response Evaluation Criteria in Solid Tumours
- ORR:
-
Objective response rates
- OS:
-
Overall survival
- PD:
-
Progressive disease
- PR:
-
Partial response
- RFA:
-
Radiofrequency ablation
- SD:
-
Stable disease
- (c)TACE:
-
(conventional) Transarterial chemoembolization
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 0(0):1–31. https://doi.org/10.3322/caac.21492
El-Serag HB (2011) Hepatocellular carcinoma. New Engl J Med 365:1118–1127. https://doi.org/10.1056/NEJMra1001683
Tanaka Y, Hanada K, Mizokami M, Yeo AE, Shih WK, Gojobori T, Alter HJ (2002) A comparison of the molecular clock of hepatitis C virus in the United States and Japan predicts that hepatocellular carcinoma incidence in the United States will increase over the next two decades. Proc Natl Acad Sci USA 99(24):15584–15589. https://doi.org/10.1073/pnas.242608099
Saunders D, Seidel D, Allison M, Lyratzopoulos G (2010) Systematic review: The association between obesity and hepatocellular carcinoma - Epidemiological evidence. Aliment Pharmacol Ther 31(10):1051–1063. https://doi.org/10.1111/j.1365-2036.2010.04271.x
Varela M, Real MI, Burrel M, Forner A, Sala M, Brunet M, Ayuso C, Castells L, et al. (2007) Chemoembolization of hepatocellular carcinoma with drug eluting beads: Efficacy and doxorubicin pharmacokinetics. J Hepatol 46(3):474–481. https://doi.org/10.1016/j.jhep.2006.10.020
Llovet JM, Brú C, Bruix J (1999) Prognosis of Hepatocellular Carcinoma: The BCLC Staging Classification. Semin Liver Dis 19(3):329–338. https://doi.org/10.1055/s-2007-1007122
Vogel A, Cervantes A, Chau I, Daniele B, LLovet JM, Meyer T, Nault JC, et al. (2018) Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up† Ann Oncol 29(4):iv238-iv255. https://doi.org/10.1093/annonc/mdy308
Greten TF, Malek NP, Schmidt S, Arends J, Bartenstein P, Bechstein W, et al. (2008) Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft, Deutsche Krebshilfe, AWMF): Diagnostik und Therapie des hepatozellulären Karzinoms, Langversion 1.0. AWMF Regist 032-053OL 2013:1–153
Llovet JM, Bruix J (2008) Novel advancements in the management of hepatocellular carcinoma in 2008. J Hepatol 48:20–37. https://doi.org/10.1016/j.jhep.2008.01.022
Burrel M, Reig M, Forner A, Barrufet M, de Lope CR, Tremosini S, Ayuso C, LLovet JM (2012) Survival of patients with hepatocellular carcinoma treated by transarterial chemoembolisation (TACE) using Drug Eluting Beads. Implications for clinical practice and trial design. J Hepatol 56:1330–1335. https://doi.org/10.1016/j.jhep.2012.01.008
Meropol NJ, Weinfurt KP, Burnett CB, Balshem A, Benson AB, Castel L, et al. (2003) Perceptions of patients and physicians regarding phase I cancer clinical trials: Implications for physician-patient communication. J Clin Oncol 21:2589–2596. https://doi.org/10.1200/JCO.2003.10.072
Sun V, Ferrell B, Juarez G, Wagman LD, Yen Y, Chung V (2008) Symptom Concerns and Quality of Life in Hepatobiliary Cancers. Oncol Nurs Forum 35(3):E45–E52. https://doi.org/10.1188/08.ONF.E45-E52
Ghani MA, Thakur V, Geschwind JF (2017) Choice of Intra-arterial Therapy for Hepatocellular Carcinoma: Evidence and Future Horizons. Dig Dis Interv 1(2):105-114. https://doi.org/10.1055/s-0037-1603891
Poon RT, Tso WK, Pang RW, Ng KK, Woo R, Shing K, Fan ST (2007) A Phase I/II Trial of Chemoembolization for Hepatocellular Carcinoma Using a Novel Intra-Arterial Drug-Eluting Bead. Clin Gastroenterol Hepatol 5(9):1100–1108. https://doi.org/10.1016/j.cgh.2007.04.021
Lammer J, Malagari K, Vogl T, Pilleul F, Denys A, Watkinson A, et al. (2010) Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: Results of the PRECISION V study. Cardiovasc Intervent Radiol 33:41–52. https://doi.org/10.1007/s00270-009-9711-7
Malagari K, Pomoni M, Moschouris H, Kelekis A, Charokopakis A, Bouma E (2014) Chemoembolization of Hepatocellular Carcinoma with Hepasphere 30–60 µm. Safety and Efficacy Study. Cardiovasc Interv Radiol 37:165–175. https://doi.org/10.1007/s00270-013-0777-x
Namur J, Citron SJ, Sellers MT, Dupuis MH, Wassef M, Manfait M, Laurent A (2011) Embolization of hepatocellular carcinoma with drug-eluting beads: Doxorubicin tissue concentration and distribution in patient liver explants. J Hepatol 55:1332–1338. https://doi.org/10.1016/j.jhep.2011.03.024
Prajapati HJ, Xing M, Spivey JR, Hanish SI, El-Rayes BF, Kauh JS, Chen Z, Kim HS (2014) Survival, efficacy, and safety of small versus large doxorubicin drug-eluting beads TACE chemoembolization in patients with unresectable HCC. Am J Roentgenol 203:W706–W714. https://doi.org/10.2214/AJR.13.12308
Padia SA, Shivaram G, Bastawrous S, Bhargava P, Vo NJ, Vaidya S, et al. (2013) Safety and efficacy of drug-eluting bead chemoembolization for hepatocellular carcinoma: Comparison of small-versus medium-size particles. J Vasc Interv Radiol 24:301–306. https://doi.org/10.1016/j.jvir.2012.11.023
Wáng Y-X, De Baere T, Idée J-M, Ballet S (2015) Transcatheter embolization therapy in liver cancer: an update of clinical evidences. Chin J Cancer Res 27(2):96–121. https://doi.org/10.3978/j.issn.1000-9604.2015.03.03
Gupta S, Wright KC, Ensor J, Van Pelt CS, Dixon KA, Kundra V (2011) Hepatic Arterial Embolization with Doxorubicin-Loaded Superabsorbent Polymer Microspheres in a Rabbit Liver Tumor Model. Cardiovasc Interv Radiol 34:1021–1030. https://doi.org/10.1007/s00270-011-0154-6
Dhanasekaran R, Kooby DA, Staley CA, Kauh JS, Khanna V, Kim HS (2010) Prognostic factors for survival in patients with unresectable hepatocellular carcinoma undergoing chemoembolization with doxorubicin drug-eluting beads: a preliminary study. HPB 12:174–180. https://doi.org/10.1111/j.1477-2574.2009.00138.x
Deipolyi AR, Oklu R, Al-Ansari S, Zhu AX, Goyal L, Ganguli S (2015) Safety and efficacy of 70-150 μm and 100-300 μm drug-eluting bead transarterial chemoembolization for hepatocellular carcinoma. J Vasc Interv Radiol 26(4):516–522. https://doi.org/10.1016/j.jvir.2014.12.020
Brown KT, Do RK, Gonen M, Covey AM, Getrajdman GI, Sofocleous CT, et al. (2016) Randomized trial of hepatic artery embolization for hepatocellular carcinoma using doxorubicin-eluting microspheres compared with embolization with microspheres alone. J Clin Oncol 34(17):2046–2053. https://doi.org/10.1200/JCO.2015.64.0821
Lencioni R, de Baere T, Burrel M, Caridi JG, Lammer J, Malagari K, et al. (2012) Transcatheter Treatment of Hepatocellular Carcinoma with Doxorubicin-loaded DC Bead (DEBDOX): Technical Recommendations. Cardiovasc Intervent Radiol 35:980–985. https://doi.org/10.1007/s00270-011-0287-7
Galle PR, Forner A, LLovet JM, Mazzaferro V, Piscaglia F, Raoul JL, et al. (2018) EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 69:182–236. https://doi.org/10.1016/j.jhep.2018.03.019
Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, et al. (2015) Assessment of Liver Function in Patients With Hepatocellular Carcinoma: A New Evidence-Based Approach — The ALBI Grade. J Clin Oncol 33(6):550-558. https://doi.org/10.1200/JCO.2014.57.9151
Wiesner RH, Freeman RB, Mulligan DC (2004) Liver transplantation for hepatocellular cancer: The impact of the MELD allocation policy. Gastroenterology 127(5):261-267. https://doi.org/10.1053/j.gastro.2004.09.040
Sun JH, Zhou GH, Zhang YL, Nie CH, Zhou TY, Ai J, et al. (2017) Chemoembolization of liver cancer with drug-loading microsphere 50-100 µm. Oncotarget 8(3):5392–5399. https://doi.org/10.18632/oncotarget.14281
Hinrichs JB, Hasdemir DB, Nordlohne M, Schweitzer N, Wacker F, Vogel A, Kirstein MM, Marquardt S, Rodt T (2017) Health-Related Quality of Life in Patients with Hepatocellular Carcinoma Treated with Initial Transarterial Chemoembolization. Cardiovasc Intervent Radiol 40(10):1559–1566. https://doi.org/10.1007/s00270-017-1681-6
Kirchner T, Marquardt S, Werncke T, Kirstein MM, Brunkhorst T, Wacker F, et al. (2018) Comparison of health-related quality of life after transarterial chemoembolization and transarterial radioembolization in patients with unresectable hepatocellular carcinoma. Abdom Radiol 44(4):1554-1561. https://doi.org/10.1007/s00261-018-1802-y
Hartrumpf KJ, Marquardt S, Werncke T, Murray T, Kirstein MM, Vogel A, et al. (2018) Quality of life in patients undergoing repetitive TACE for the treatment of intermediate stage HCC. J Cancer Res Clin Oncol 144(10):1991–1999. https://doi.org/10.1007/s00432-018-2704-7
Boulin M, Delhom E, Pierredon-Foulongne MA, Cercueil JP, Guiu B (2015) Transarterial chemoembolization for hepatocellular carcinoma: An old method, now flavor of the day. Diagn Interv Imaging 96(6):607–615. https://doi.org/10.1016/j.diii.2015.04.005
Blazeby JM, Currie E, Zee BC, Chie WC, Poon RT, Garden OJ (2004) Development of a questionnaire module to supplement the EORTC QLQ-C30 to assess quality of life in patients with hepatocellular carcinoma, the EORTC QLQ-HCC18. Eur J Cancer 40(16):2439–2444. https://doi.org/10.1016/j.ejca.2004.06.033
Chie WC, Blazeby JM, Hsiao CF, Chiu HC, Poon RT, Mikoshiba N, et al. (2012) International cross-cultural field validation of an European Organization for Research and Treatment of Cancer questionnaire module for patients with primary liver cancer, the European Organization for Research and Treatment of Cancer quality-of-life Questionnaire HCC18. Hepatology 55(4):1122–1129. https://doi.org/10.1002/hep.24798
Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ (1993) The European Organisation for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Nat Cancer Inst 85(5):365-376. https://doi.org/10.1093/jnci/85.5.365
Shim JH, Lee HC, Kim SO, Shin YM, Kim KM, Lim YS, et al. (2012) Which Response Criteria Best Help Predict Survival of Patients with Hepatocellular Carcinoma Following Chemoembolization? A Validation Study of Old and New Models. Radiology 262(2):708–718. https://doi.org/10.1148/radiol.11110282
Sacks D, McClenny TE, Cardella JF, Lewis CA (2003) Society of Interventional Radiology Clinical Practice Guidelines. J Vasc Interv Radiol 14(9):199-202. https://doi.org/10.1097/01.RVI.0000094584.83406.3e
Omary RA, Bettmann MA, Cardella JF, Bakal CW, Schwartzberg MS, Sacks D, et al. (2003) Quality Improvement Guidelines for the Reporting and Archiving of Interventional Radiology Procedures. J Vasc Interv Radiol. 14(9):293–295. https://doi.org/10.1097/01.RVI.0000094601.83406.e1
Boulin M, Hillon P, Cercueil JP, Bonnetain F, Dabakuyo S, Minello A, et al. (2014) Idarubicin-loaded beads for chemoembolisation of hepatocellular carcinoma: results of the IDASPHERE phase I trial. Aliment Pharmacol Ther 39(11):1301-1313. https://doi.org/10.1111/apt.12746
Bilbao JI, de Luis E, García de Jalón JA, De Martino A, Lozano MD, et al. (2008) Comparative Study of Four Different Spherical Embolic Particles in an Animal Model: A Morphologic and Histologic Evaluation. J Vasc Interv Radiol 19(11):1625–1638. https://doi.org/10.1016/j.jvir.2008.07.014
Duan F, Wang EQ, Lam MG, Abdelmaksoud MH, Louie JD, Hwang GL, et al. (2016) Superselective Chemoembolization of HCC: Comparison of Short-term Safety and Efficacy between Drug-eluting LC Beads, QuadraSpheres, and Conventional Ethiodized Oil Emulsion. Radiology 278(2):612–621. https://doi.org/10.1148/radiol.2015141417
Reyes DK, Vossen JA, Kamel IR, Azad NS, Wahlin TA, Torbenson MS, et al. (2009) Single-center phase II trial of transarterial chemoembolization with drug-eluting beads for patients with unresectable hepatocellular carcinoma: initial experience in the United States. Cancer J 15(6):526–532. https://doi.org/10.1097/PPO.0b013e3181c5214b
Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, et al. (2017) Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 389:56–66. https://doi.org/10.1016/S0140-6736(16)32453-9
Malagari K, Pomoni M, Spyridopoulos TN, Moschouris H, Kelekis A, Spyros D, et al. (2011) Safety Profile of Sequential Transcatheter Chemoembolization with DC Bead: Results of 237 Hepatocellular Carcinoma (HCC) Patients. Cardiovasc Interv Radiol 34(4):774–785. https://doi.org/10.1007/s00270-010-0044-3
Lee K-H, Liapi E, Vossen JA, Buijs M, Ventura VP, Georgiades C, et al. (2008) Distribution of Iron Oxide-containing Embosphere Particles after Transcatheter Arterial Embolization in an Animal Model of Liver Cancer: Evaluation with MR Imaging and Implication for Therapy. J Vasc Interv Radiol 19(10):1490–1496. https://doi.org/10.1016/j.jvir.2008.06.008
Lewis AL, Taylor RR, Hall B, Gonzalez MV, Willis SL, Stratford PW (2006) Pharmacokinetic and Safety Study of Doxorubicin-eluting Beads in a Porcine Model of Hepatic Arterial Embolization. J Vasc Interv Radiol 17(8):1335–1343. https://doi.org/10.1097/01.RVI.0000228416.21560.7F
Vincenzi B, Di Maio M, Silletta M, D’Onofrio L, Spoto Chiara, Piccirillo MC, et al. (2015) Prognostic Relevance of Objective Response According to EASL Criteria and mRECIST Criteria in Hepatocellular Carcinoma Patients Treated with Loco-Regional Therapies: A Literature-Based Meta-Analysis. PLoS One 10(7):e0133488. https://doi.org/10.1371/journal.pone.0133488
Lencioni R, Montal R, Torres F, Park JW, Decaens T, Raoul JL, et al. (2017) Objective response by mRECIST as a predictor and potential surrogate end-point of overall survival in advanced HCC. J Hepatol 66(6):1166–1172. https://doi.org/10.1016/j.jhep.2017.01.012
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors of this manuscript declare relationships with the following companies: Siemens Healthcare and ProMedicus (Bernhard Meyer and Frank Wacker; outside the submitted work). The remaining authors declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Grumme, J., Werncke, T., Meine, T.C. et al. Transarterial chemoembolization for hepatocellular carcinoma: quality of life, tumour response, safety and survival comparing two types of drug-eluting beads. Abdom Radiol 45, 3326–3336 (2020). https://doi.org/10.1007/s00261-019-02349-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-019-02349-w