Abstract
Purpose
To assess the diagnostic effectiveness of arterial spin labeling (ASL) MR imaging in differentiating fat-poor AML from clear cell renal cell carcinoma (ccRCC).
Methods
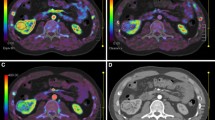
In this prospective study, 29 ccRCC patients and 9 fat-poor AML patients underwent routine anatomical MRI and ASL at 3T before surgery after signing written informed consent form. For each tumor, tumor blood flow (TBF) was measured in a region of interest (ROI) which was positioned to outline the edge of the target lesions on ASL perfusion maps. Additionally, the mean TBF values were obtained by standardizing the TBF using a blood flow measurement in the reference ROI. Moreover, a cluster containing more than 10 voxels was chosen from the renal cortex and medulla area in normal contralateral kidney as a reference ROI to calculate tumor-to-cortex ratio and tumor-to-medulla ratio. Independent sample t test was used to examine the alteration among the groups of fat-poor AML and ccRCC. ASL images were together analyzed by two radiologists to assess the following characteristics of the renal mass: predominant SI in the tumor on ASL images was lower than, as same as, or higher than SI of the cortex. For qualitative variables, Fisher’s exact test was employed to compare the proportions of these two groups. The sensitivity, specificity ,and accuracy required for discrimination of fat-poor AML from ccRCC were quantified using receiver operating characteristic (ROC) curve. The corresponding optimal cutoff value was obtained for each parameter as well.
Results
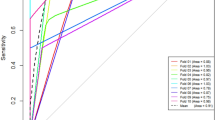
The TBF value was significantly higher in ccRCC group than that in fat-poor AML (270.49 ± 78.88 ml/100 g/min vs. 146.68 ± 47.21 ml/100 g/min; P < 0.01). Both tumor-to-cortex and tumor-to-medulla ratios were notably higher in ccRCC group compared with those in fat-poor AML group (1.22 ± 0.26 vs. 0.74 ± 0.14, 3.13 ± 0.94 vs. 1.77 ± 0.55; P < 0.05). The values of area under the ROC curve (AUC) for TBF, tumor-to-cortex ratio, and tumor-to-medulla ratio were 0.931, 0.964, and 0.900, respectively. No significant difference in AUC values among these three measurements was observed. For qualitative variables, the SI of fat-poor AML was equal to or slightly lower than that of renal medulla and the SI of ccRCC was found to be higher than renal cortex in ASL.
Conclusion
ASL MRI performs well in differentiating fat-poor AML from ccRCC in both qualitative and quantitative analyses.







Similar content being viewed by others
References
ME Snyder, A Bach, MW Kattan, GV Raj, VE Reuter, P Russo. Incidence of benign lesions for clinically localized renal masses smaller than 7 cm in radiological diameter: influence of sex. The Journal of urology. 2006;176(null):2391-5; discussion 5-6. https://doi.org/10.1016/j.juro.2006.08.013
Schachter LR, Cookson MS, Chang SS, et al. Second prize: frequency of benign renal cortical tumors and histologic subtypes based on size in a contemporary series: what to tell our patients. Journal of Endourology. 2007;21(8):819. https://doi.org/10.1089/end.2006.9937
Y Fujii, Y Komai, K Saito, et al. Incidence of benign pathologic lesions at partial nephrectomy for presumed RCC renal masses: Japanese dual-center experience with 176 consecutive patients. Urology. 2008;72(3):598-602. https://doi.org/10.1016/j.urology.2008.04.054
Richard PO, Jewett MAS, Bhatt JR, et al. Renal Tumor Biopsy for Small Renal Masses: A Single-center 13-year Experience. European urology. 2015;68(6):1007-13. https://doi.org/10.1016/j.eururo.2015.04.004
Remzi M, Özsoy M, Klingler HC, et al. Are Small Renal Tumors Harmless? Analysis of Histopathological Features According to Tumors 4 Cm or Less in Diameter. Journal of Urology. 2006;176(3):896-9. https://doi.org/10.1016/j.juro.2006.04.047
Pahernik S, Ziegler S, Roos F, Melchior SW, Thüroff JW. Small renal tumors: correlation of clinical and pathological features with tumor size. J Urol. 2007;178(2):414-7. https://doi.org/10.1016/j.juro.2007.03.129
K Sasiwimonphan, N Takahashi, BC Leibovich, RE Carter, TD Atwell, A Kawashima. Small (< 4 cm) renal mass: differentiation of angiomyolipoma without visible fat from renal cell carcinoma utilizing MR imaging. Radiology. 2012;263(1):160-8. https://doi.org/10.1148/radiol.12111205
Katabathina VS, Raghunandan V, Nagar AM, Pheroze T, Menias CO, Prasad SR. Mesenchymal neoplasms of the kidney in adults: imaging spectrum with radiologic-pathologic correlation. Radiographics A Review Publication of the Radiological Society of North America Inc. 2010;30(6):1525-40. https://doi.org/10.1148/rg.306105517
Schieda N, Avruch L, Flood TA. Small (< 1 cm) incidental echogenic renal cortical nodules: chemical shift MRI outperforms CT for confirmatory diagnosis of angiomyolipoma (AML). Insights Into Imaging. 2014;5(3):295-9. https://doi.org/10.1007/s13244-014-0323-7
Burdeny DA, Semelka RC, Kelekis NL, Reinhold C,., Ascher SM. Small (< 1.5 cm) angiomyolipomas of the kidney: characterization by the combined use of in-phase and fat-attenuated MR techniques. Magnetic Resonance Imaging. 1997;15(2):141-5. https://doi.org/10.1016/S0730-725X(96)00370-0
Sherman JL, Hartman DS, Friedman AC, Madewell JE, Davis CJ, Goldman SM. Angiomyolipoma: computed tomographic-pathologic correlation of 17 cases. AJR American journal of roentgenology. 1981;137(6):1221-6. https://doi.org/10.2214/ajr.137.6.1221
L Richmond, M Atri, C Sherman, S Sharir. Renal cell carcinoma containing macroscopic fat on CT mimics an angiomyolipoma due to bone metaplasia without macroscopic calcification. The British journal of radiology. 2010;83(992):e179-81. https://doi.org/10.1259/bjr/46452134
Israel GM, Nicole H, Elizabeth H, Glenn K. The use of opposed-phase chemical shift MRI in the diagnosis of renal angiomyolipomas. AJR American journal of roentgenology. 2005;184(6):1868-72. https://doi.org/10.2214/ajr.184.6.01841868
Zhang YY, Luo S, Liu Y, Xu RT. Angiomyolipoma with minimal fat: Differentiation from papillary renal cell carcinoma by helical CT. Clinical Radiology. 2013;68(4):365-70. https://doi.org/10.1016/j.crad.2012.08.028
Sant GR, Heaney JA, Ucci AA, Sarno RC, Meares EM. Computed tomographic findings in renal angiomyolipoma: an histologic correlation. Urology. 1984;24(3):293-6. https://doi.org/10.1016/0090-4295(84)90365-0
Jeong Kon K, Soo-Youn P, Jeong-Hee S, Kyoung-Sik C. Angiomyolipoma with minimal fat: differentiation from renal cell carcinoma at biphasic helical CT. Radiology. 2004;230(3):677-84. https://doi.org/10.1148/radiol.2303030003
Claus S, Herts BR, Motta-Ramirez GA, et al. Angiomyolipoma with minimal fat on MDCT: can counts of negative-attenuation pixels aid diagnosis? AJR American journal of roentgenology. 2009;192(2):438-43. https://doi.org/10.2214/ajr.08.1180
Yeon KJ, Jeong Kon K, Namkug K, Kyoung-Sik C. CT histogram analysis: differentiation of angiomyolipoma without visible fat from renal cell carcinoma at CT imaging. Radiology. 2008;246(2):472-9. https://doi.org/10.1148/radiol.2462061312
M Jinzaki, A Tanimoto, Y Narimatsu, et al. Angiomyolipoma: imaging findings in lesions with minimal fat. Radiology. 1997;205(2):497-502. https://doi.org/10.1148/radiology.205.2.9356635
Jeong Kon K, Soo Hyun K, Yoon Jin J, et al. Renal angiomyolipoma with minimal fat: differentiation from other neoplasms at double-echo chemical shift FLASH MR imaging. Radiology. 2006;239(1):174-80. https://doi.org/10.1148/radiol.2391050102
Nicole H, Long N, Genega EM, et al. Angiomyolipoma with minimal fat: can it be differentiated from clear cell renal cell carcinoma by using standard MR techniques? Radiology. 2012;265(2):468-77. https://doi.org/10.1148/radiol.12112087
Mesut R, Mehmet O, Hans-Christoph K, et al. Are small renal tumors harmless? Analysis of histopathological features according to tumors 4 cm or less in diameter. Journal of Urology. 2006;176(3):896-9. https://doi.org/10.1016/j.juro.2006.04.047
Roberts DA, Detre JA, Bolinger L,., et al. Renal perfusion in humans: MR imaging with spin tagging of arterial water. Radiology. 1995;196(1):281-6. https://doi.org/10.1148/radiology.196.1.7784582
Alsop DC, Detre JA. Multisection cerebral blood flow MR imaging with continuous arterial spin labeling. Radiology. 1998;208(2):410-6. https://doi.org/10.1148/radiology.208.2.9680569
Lanzman RS, Wittsack HJ, Martirosian P, et al. Quantification of renal allograft perfusion using arterial spin labeling MRI: initial results. European Radiology. 2010;20(6):1485-91. https://doi.org/10.1007/s00330-009-1675-0
Michael F, Petros M, Juergen L, et al. Perfusion MR imaging with FAIR true FISP spin labeling in patients with and without renal artery stenosis: initial experience. Radiology. 2006;238(3):1013-21. https://doi.org/10.1148/radiol.2382041623
L Maccotta, JA Detre, DC Alsop. The efficiency of adiabatic inversion for perfusion imaging by arterial spin labeling. NMR in biomedicine. 1997;10(null):216-21. https://doi.org/10.1002/(SICI)1099-1492(199706/08)10:4/5%3c216::AID-NBM468%3e3.0.CO;
Lanzman RS, Robson PM, Sun MR, et al. Arterial spin-labeling MR imaging of renal masses: correlation with histopathologic findings. Radiology. 2012;265(3):799. https://doi.org/10.1148/radiol.12112260
BR Lane, H Aydin, TL Danforth, et al. Clinical correlates of renal angiomyolipoma subtypes in 209 patients: classic, fat poor, tuberous sclerosis associated and epithelioid. The Journal of urology. 2008;180(3):836-43. https://doi.org/10.1016/j.juro.2008.05.041
Jason H, Fogarty JD, Hoenig DM, Maomi L, Robert B, Reza G. Imaging characteristics of minimal fat renal angiomyolipoma with histologic correlations. Urology. 2005;66(6):1155-9. https://doi.org/10.1016/j.urology.2005.06.119
Milner J, Mcneil BJ, Proud K, et al. Fat poor renal angiomyolipoma: patient, computerized tomography and histological findings. Journal of Urology. 2006;176(3):905-9. https://doi.org/10.1016/j.juro.2006.04.016
Petros M, Uwe K, Irina M, Fritz S. FAIR true-FISP perfusion imaging of the kidneys. Magnetic Resonance in Medicine. 2004;51(2):353–61. https://doi.org/10.1002/mrm.10709
RL Wolf, J Wang, S Wang, et al. Grading of CNS neoplasms using continuous arterial spin labeled perfusion MR imaging at 3 Tesla. Journal of magnetic resonance imaging : JMRI. 2005;22(4):475-82. https://doi.org/10.1002/jmri.20415
Zhang Y, Kapur P, Yuan Q, et al. Tumor Vascularity in Renal Masses: Correlation ofArterial Spin-Labeled and Dynamic Contrast-Enhanced Magnetic Resonance Imaging Assessments. Clinical Genitourinary Cancer. 2016;14(1):e25-e36. https://doi.org/10.1016/j.clgc.2015.08.007
Ching BC, Tan HS, Tan PH, et al. Differential radiologic characteristics of renal tumours on multiphasic computed tomography. Singapore Medical Journal. 2016;58(5). https://doi.org/10.11622/smedj.2016081
Mi-Hyun K, Jungbok L, Gyunggoo C, Kyoung-Sik C, Jungmi K, Jeong Kon K. MDCT-based scoring system for differentiating angiomyolipoma with minimal fat from renal cell carcinoma. Acta Radiologica. 2013;54(10):1201-9. https://doi.org/10.1177/0284185113491087
Ding Y, Zeng M, Rao S, Chen C, Fu C, Zhou J. Comparison of Biexponential and Monoexponential Model of Diffusion-Weighted Imaging for Distinguishing between Common Renal Cell Carcinoma and Fat Poor Angiomyolipoma. Korean Journal of Radiology. 2016;17(6):853-63. https://doi.org/10.3348/kjr.2016.17.6.853
Feng Z, Rong P, Cao P, et al. Machine learning-based quantitative texture analysis of CT images of small renal masses: Differentiation of angiomyolipoma without visible fat from renal cell carcinoma. European Radiology. 2018;28(4):1625-33. https://doi.org/10.1007/s00330-017-5118-z
Wintermark M, Sesay M, Barbier E, et al. Comparative overview of brain perfusion imaging techniques. Journal of Neuroradiology. 2005;32(5):294-314. https://doi.org/10.1161/01.STR.0000177839.03321.25
Funding
National Natural Science Foundation of China, 81401384. Social Develop Foundation of Yangzhou, 2017066. Yangzhou City Science and Education Strengthening Leading Talents Project, LJRC201810. Yangzhou City Science and Education Strengthening Key Talents Project, ZDRC201873. Jiangsu Province “Six First Project” for High-Level Health Professionals, LGY2019032.
Author information
Authors and Affiliations
Contributions
Ye J is the guarantor of integrity of the entire study; all authors contributed to the approval of final version of submitted manuscript and agree to ensure that any questions related to the work are appropriately resolved; Zheng J contributed to clinical case studies; Wang SA contributed to statistical analysis. All authors contributed to manuscript editing.
Corresponding author
Ethics declarations
Conflicts of interest
All authors declare that they have no any conflict of interest.
Informed consent
Informed consent was obtained from the patient included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ye, J., Xu, Q., Wang, SA. et al. Differentiation between fat-poor angiomyolipoma and clear cell renal cell carcinoma: qualitative and quantitative analysis using arterial spin labeling MR imaging. Abdom Radiol 45, 512–519 (2020). https://doi.org/10.1007/s00261-019-02303-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-019-02303-w