Abstract
Purpose
Peri-tumoral hyperintensity (P-hyperintensity) is occasionally seen in hepatocellular carcinoma (HCC) on the hepatobiliary (HB) phase of gadoxetic acid-enhanced MRI (EOB-MRI). A recent study reported peri-tumoral hyperplasia (P-hyperplasia) associated with over-expression of glutamine synthetase (GS) in HCC or metastatic carcinoma. The aim of this study was to analyze the correlation between P-hyperintensity on the HB phase and GS expression indicating P-hyperplasia and reveal its pathological features.
Methods
Seventy-seven surgically resected HCCs from 68 patients were analyzed. The grade of P-hyperintensity on HB phase was divided according to the degree of the peri-tumoral hyperintense signal: grade 0 (no P-hyperintensity), grade 1 (less than 50% of the tumor border), grade 2 (50%–80%), grade 3 (80%–100%). Immunohistochemical staining for GS and organic anion transporter polypeptides (OATP)1B3 was performed. The relationships among P-hyperplasia (peri-tumoral GS expression) and OATP1B3 expression, P-hyperintensity, and pathological features of the tumor were analyzed.
Results
Thirty-four HCCs were classified as P-hyperintensity grade 0, 29 HCCs as grade 1,10 nodules as grade 2, and 4 HCCs as grade 3. P-hyperplasia was observed in 3/34 (8.8%) P-hyperintensity grade 0, 16/29 (55.2%) grade 1, 9/10 (90%) grade 2, and 4/4 (100%) grade 3. The incidence of P-hyperplasia was significantly increased in P-hyperintensity grades 1–3 compared with grade 0 (p < 0.0001). Hepatocytes in all P-hyperplasia sites demonstrated definite OATP1B3 expression. Microscopic hepatic venous invasion was significantly increased in P-hyperintensity-positive HCCs compared with negative HCCs (p = 0.0017).
Conclusions
P-hyperintensity on HB phase in HCC may indicate p-hyperplasia with GS and OATP1B3 expression and a higher incidence of microscopic hepatic venous invasion.
Similar content being viewed by others
Gadoxetic acid (gadolinium ethoxybenzyl diethylenetriaminepentaacetic acid; Gd-EOB-DTPA; Primovist ® (sold as Eovist ® in the USA), Bayer Schering Pharma AG, Berlin, Germany) is a contrast agent for magnetic resonance (MR) imaging with hepatocyte-specific property [1, 2]. Gadoxetic acid-enhanced MRI (EOB-MRI) has been becoming more important in the detection and characterization of hepatic mass lesions including hepatocellular carcinoma (HCC) and other hepatic nodules [3,4,5,6,7,8].
Recent studies have revealed the transporter mechanism of gadoxetic acid in HCCs. Sinusoidal membranous uptake transporter organic anion-transporting polypeptide (OATP)1B3 (synonymous with OATP8) expression in HCC cells determines the signal intensity of HCC on the hepatobiliary (HB) phase of EOB-MRI [9, 10]. In addition, benign hepatocellular nodules including hepatocellular adenoma (HCA), focal nodular hyperplasia, and dysplastic nodule also show a positive correlation between signal intensity on HB phase of EOB-MRI and OATP1B3 expression [11,12,13,14,15,16]. Therefore, OATP1B3 is thought to be the main uptake transporter of gadoxetic acid determining the signal intensity on HB phase in hepatocellular nodules including hyperplastic lesions.
In addition, the molecular background of OATP1B3 expression has been becoming clearer. In normal liver parenchyma, OATP1B3 and glutamine synthetase (GS), the downstream target of the beta-catenin signaling, are considered to be expressed by the interaction of Wnt/β-catenin signaling and hepatocyte nuclear factor (HNF) 4α in peri-central (zone 3) hepatocytes [17,18,19,20]. Therefore, co-expression of GS and OATP1B3 is seen in peri-central hepatocytes compared with hepatocytes surrounding the portal venule [17,18,19,20]. In HCC, an intense correlation between beta-catenin mutation with GS expression and OATP1B3 expression was also reported [21, 22], and HCC showing hyperintensity relative to the surrounding liver (OATP1B3 over-expressed HCC) on HB phase was confirmed to be associated with activation of β-catenin signaling, GS, and HNF4α [23, 24]. Moreover, β-catenin-activated HCA shows over-expression of GS and OATP1B3 resulting in iso/hyperintensity on HB phase of EOB-MRI [12, 13]. Therefore, an intense correlation between OATP1B3, which is the most important determinant of Gd-EOB-DTPA uptake into hepatocytes and/or HCC cells, and GS expression has been strongly suggested.
In our experience, peri-tumoral hyperintensity (P-hyperintensity) surrounding a HCC nodule is occasionally seen on HB phase of EOB-MRI. However, the mechanism of P-hyperintensity is still unknown. On the other hand, a recent study showed peri-tumoral hyperplasia (P-hyperplasia) of hepatocytes associated with over-expression of GS surrounding HCC or metastatic carcinoma [25]. It was defined as a peri-tumoral rim of hyperplastic hepatocytes with altered plate architecture (including hepatocyte rosettes or thickened plates) delimited by a region of parenchymal atrophy and with diffuse hepatocellular expression of GS and variable sinusoidal CD34 expression, which was surmised to be due to peri-tumoral portal blood flow obstruction.
Based on these facts and previous reports, we speculated that the P-hyperintensity on HB phase of EOB-MRI might be due to P-hyperplasia with higher expression of GS and OATP1B3 and might have some relation with the biological nature of HCC.
The aim of this study was to analyze the correlation between P-hyperintensity on HB phase of EOB-MRI and immunohistochemical expression of GS indicating P-hyperplasia in the liver parenchyma surrounding HCC, and its relation with pathological features of HCCs.
Materials and methods
Patients
This retrospective study received the approval of our institutional ethics committee. Firstly, we included 86 consecutive surgically resected patients confirmed as HCC pathologically in our hospital during the period from 2008 to 2010. And we excluded 16 patients because EOB-MRI was not performed. In addition, two patients were excluded because of poor quality of HB phase image. We finally included 77 HCCs from 68 patients in our hospital during the period from 2008 to 2010. Mean patient age was 65.2 years ± 9.73 (standard deviation) (range 38–81 years) for the entire study group. The male/female ratio was 53:24, and 68 of 77 HCCs had a background of chronic hepatitis or liver cirrhosis. The level of serum AFP was the following: normal level of serum AFP (< 10 ng/mL) (n = 23), 10–100 ng/mL (n = 29), more than 100 ng/mL (n = 25). And Child–pugh score was the following: Child–pugh score A (n = 71), B (n = 6) (Table 1).
Imaging details
MR images were obtained with a 1.5/3.0 Tesla MR system (Signa HDx; GE Medical Systems, Milwaukee, WI). The protocols for T1-weighted, T2-weighted, diffusion-weighted imaging and contrast material-enhanced T1-weighted imaging are shown in Table 2. T1-weighted imaging was performed during precontrast and hepatobiliary phase. T2-weighted imaging was obtained after the dynamic study. A dose of 0.1 mL/kg of gadoxetic acid was intravenously injected at a flow rate of 1.0 mL/s, followed by a 30-mL saline flush. We employed the test injection method (1.5 mL of gadoxetic acid plus an 8-mL saline flush) to determine the optimal arterial dominant phase, which was determined as the peak time of enhancement in the abdominal aorta plus an additional 10 s and half of imaging time in the arterial phase. Portal phase and equilibrium phase images were obtained at 60–90 s and 120–180 s after injection, respectively. The HB phase was obtained 20 min after the injection in all cases. The mean interval between surgery and EOB-MRI was 50.3 days.
Contrast-enhanced dynamic CT images were obtained with CT systems (VCT64; GE Medical Systems, Milwaukee, Wis, or Somatom Definition Flash; Siemens, Erlangen, Germany) with the following parameters: voltage, 120 kV; amperage, 300–350 mA; rotation period, 0.5 s; detector collimation, 0.6–0.625 mm; pitch, 0.516–1.0; and section thickness, 2.5–3.0 mm. 100–150 mL of contrast material with 600 mg of iodine per kilogram (Iopamiron; Bayer Schering Pharma, Berlin; Iomeron; Eisai, Tokyo, Japan; Omnipaque; Daiichi-Sankyo, Tokyo, Japan; or Optiray; Mallinckrodt Japan, Tokyo, Japan) was injected for 30 s after precontrast scanning. The equilibrium phase was obtained after a scanning delay of 150 s. The mean interval between surgery and dynamic CT was 65.7 days.
Imaging analysis
We just defined P-hyperintensity as hyperintensity rim surrounding the tumor. On HB phase of EOB-MRI, the fibrous capsule is not evaluated clearly. If there is a hypointense rim surrounding the tumor suggesting a fibrous capsule, we evaluated the outside of the capsule like rim when we evaluated P-hyperintensity.
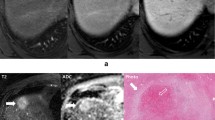
HCCs were grouped according to the degree of the peri-tumoral hyperintense signal at the level of the largest diameter of the tumor on the HB phase of EOB-MRI: grade 0 (no P-hyperintensity), grade 1 (P-hyperintensity was seen on less than 50% of the tumor border), grade 2 (50%–80%), grade 3 (80%–100%) (Fig. 1). We also evaluated the presence of peri-tumoral hypointensity surrounding the tumor on HB phase of EOB-MRI as reported previously [26].
P-hyperintensity grading on HB phase of EOB-MRI. A Patients were grouped according to the degree of the peri-tumoral hyperintense signal on the HB phase of EOB-MRI: grade 0 (no P-hyperintensity), grade 1 (P-hyperintensity was seen on less than 50% of the tumor border), grade 2 (50%–80%), grade 3 (80%–100%). B. Grade 0 (no P-hyperintensity), there is no P-hyperintensity around a HCC on HB phase. C. Grade 1, P-hyperintensity was seen on less than 50% of the tumor border. D. Grade 2, P-hyperintensity was observed on less than 50% of the tumor border. E. Grade 3, P-hyperintensity was observed on more than 80% of the tumor border
We evaluated HCC imaging features including enhancement pattern and signal intensity on T1-weighted, T2-weighted, and diffusion-weighted imaging. As an enhancement pattern, we defined a pattern that shows arterial enhancement and washout at equilibrium phase as a typical enhancement pattern for HCC. We evaluated the arterial enhancement of the nodule on arterial dominant phase of EOB-MRI. In this analysis, hypovascular nodule was defined as a nodule showing hypointensity compared with surrounding liver on arterial dominant phase of EOB-MRI.
In the area of peri-tumoral corona enhancement on dynamic CT due to the drainage flow from hypervascular HCC, portal flow is decreased [27]. Therefore, this may have some relation to P-hyperplasia. We evaluated corona enhancement on equilibrium phase by dynamic CT. Because the transitional phase of EOB-MRI was not suitable for evaluation of corona enhancement due to the effect of gadoxetic acid uptake in the surrounding liver parenchyma. Three cases did not undergo preoperative dynamic CT imaging. Therefore, a total of 74 lesions were subjected to the analysis of corona enhancement. Corona enhancement was divided into the following three degrees: clear corona enhancement at the thickest part 3 mm or more, thin corona enhancement, and no corona enhancement.
Image analysis was performed independently by two abdominal radiologists (N.Y. with 15 years of experience and T.K. with 6 years of experience) who were blinded to the histological existence of P-hyperplasia in the nodule. When the decisions of the two radiologists differed, the final evaluation was made by consensus.
Histologic diagnosis
HCCs were diagnosed by a liver pathologist (H.I. with 16 years of experience) according to the classification proposed by the international Working Party [28], WHO classification [29], and general rules proposed by the liver cancer study group of Japan [30]. We analyzed histologic features such as the presence or absence of fibrous capsule, differentiation grade (well, moderately, and poorly differentiated), macroscopic growth patterns (indistinct margin, simple nodular, nodular type with extranodular growth, confluent multinodular infiltrative patterns), and vascular invasion including microscopic portal vein invasion and hepatic venous invasion. And liver fibrosis and inflammation of background liver was graded according to the Inuyama criteria [31]: no fibrosis (F0), portal fibrosis widening (F1), portal fibrosis widening with bridging fibrosis (F2), bridging fibrosis and lobular distortion (F3), cirrhosis (F4), none (A0), mild (A1), moderate (A2), and severe (A3) inflammation.
Immunohistochemistry
The specimens were fixed in 10% formalin after surgical removal. Immunohistochemical stainings were carried out in all nodules using the primary antibodies against human GS (mouse monoclonal, GS-6; Millipore, Billerica, Mass) and human OATP1B3 (mouse monoclonal, NB100-74482; Novus Biologicals, Littleton, Colo). One representative specimen per nodule containing the boundary between tumor and background liver was subjected to the analysis. We could not evaluate the entire tumor circumference in this study.
Definition of P-hyperplasia
Previous study revealed that P-hyperplasia lesion has diffuse immunopositivity for GS [24]. GS is present normally in hepatocytes adjacent to the hepatic venule. In this study, P-hyperplasia was defined as a rim of hyperplastic hepatocytes at least 0.5 mm thickness surrounding the tumor with higher GS expression as compared with those in non-peri-tumoral liver (Fig. 2). The membranous expression of OATP1B3 in the hepatocytes in P-hyperplasia was also evaluated as compared with that in non-peri-tumoral background liver. Immunohistochemical analysis was performed by two abdominal radiologists (N.Y. with 15 years and A.K. with 16 years of experience, both of whom are graduates of the Department of Pathology, Kanazawa University, in addition to being board certificated radiologists). When the decisions of the two observers differed, the final decision was made by consensus.
GS expression of P-hyperplasia. P-hyperplasia was defined as hyperplastic peri-tumoral hepatocytes with higher GS expression as compared with those in non-peri-tumoral liver in this study. A P-hyperplasia negative, P-hyperplasia with higher GS expression is not observed in the peri-tumoral area. (Original magnification, ×20). B P-hyperplasia positive, P-hyperplasia with higher GS expression is seen in the peri-tumoral area. (Original magnification, ×40)
Statistical analysis
Statistical significance was evaluated with software (Prism5; GraphPad Software, San Diego, Ca). The χ 2 test, Fisher’s exact test, and Mann–Whitney U test were used for the analysis of the relationship between P-hyperplasia and P-hyperintensity grade, peri-tumoral hypointensity, and their imaging and pathological analysis. P < 0.05 was considered to indicate a statistically significant difference. Inter-reader agreement was assessed by calculating a κ value or weighted κ value which was interpreted as follows: poor agreement, ≤ 0.20; fair agreement, 0.21–0.40; moderate agreement, 0.41–0.60; good agreement, 0.61–0.80; and excellent agreement, 0.81–1.00.
Results
Imaging diagnosis of HCC
In this study, 72 HCCs from 77 HCCs demonstrated typical enhancement pattern (arterial enhancement- washout) as HCC and other 5 HCCs had atypical enhancement pattern (hypovascular). 72 HCCs showing typical enhancement pattern could be diagnosed as HCC by imaging. Other imaging features of HCCs including signal intensity on T2-weighted, T1-weighted and diffusion-weighted imaging are shown in Table 3. Of 72 typical enhancement HCCs, 2 HCCs showed partial hyperintensity and 5 HCCs showed hyperintensity on HB phase of EOB-MRI. On the other hand, all atypical enhancement HCCs showed hypointensity on HB phase. Therefore, we diagnosed those atypical enhancement groups as hypovascular HCC by imaging preoperatively.
Frequency of the P-hyperintensity
In the image evaluation of the HB phase of EOB-MRI, 34 nodules (44.2%) were classified as grade 0, 29 nodules (37.7%) as grade 1,10 nodules (13.0%) as grade 2, and 4 nodules (5.2%) as grade 3. And the peri-tumoral hypointensity was observed in 27/77 (35.1%) HCCs. 18 HCCs had both P-hyperintensity and peri-tumoral hypointensity on HB phase of EOB-MRI. Neither P-hyperintensity nor peri-tumoral hypointensity was seen in 25 HCCs (32.5%). The evaluation of the P-hyperintensity grade differed between the two readers for 7 HCCs (9.1%). With visual evaluation, the weighted kappa value of the P-hyperintensity grade became 0.825. Of seven evaluation differences, 5 occurred between grades 0 and 1 and 2 occurred between grades 2 and 3 among two readers. Regarding the 5 differences of between grade 0–1, it was caused by the difference whether to take subtle findings of P-hyperintensity or not. And two differences between grades 2 and 3 occurred due to the difference in interpretation of proportion of P-hyperintensity.
Histological assessment of P-hyperplasia and correlation between P-hyperintensity and P-hyperplasia
Histologically total 32 lesions (41.6%) had P-hyperplasia showing high GS expression. The thickness of P-hyperplasia ranged from a maximum of 2.2 mm to a minimum of 0.5 mm. The relationship between P-hyperplasia and P-hyperintensity grade is shown in Fig. 3. P-hyperplasia was observed in 3/34 P-hyperintensity grade 0 nodules (8.8%), 16/29 grade 1 nodules (55.2%), 9/10 grade 2 nodules (90%), and 4/4 grade 3 nodules (100%) (Figs. 3, 4). P-hyperplasia was more frequently observed in higher P-hyperintensity grade nodules on the HB phase of EOB-MRI. There was a significant difference in the incidence of P-hyperplasia between P-hyperintensity grade 0 group and grade 1–3 group (p < 0.0001). The negative predictive value of this sign predicting P-hyperplasia was 91.2 (31/34) %. Hepatocytes with high GS expression indicating P-hyperplasia demonstrated definite membranous expression of OATP1B3 (uptake transporter of EOB) in all P-hyperplasia sites. In the normal liver, OATP1B3 expression shows zonation and is expressed predominantly in the centrilobular hepatocytes (zone 3) [32]. In P-hyperplasia sites, diffuse OATP1B3 expression was observed and the zonation of OATP1B3 expression was lost, differing from the pattern seen in non-peri-tumoral liver (Fig. 5).
Frequency of P-hyperplasia according to P-hyperintensity grade on EOB-MRI. P-hyperplasia was more frequently observed in higher P-hyperintensity grade nodules on HB phase of EOB-MRI. There was a highly significant correlation in the incidence of P-hyperplasia between P-hyperintensity grade 0 group and grade 1–3 group (p < 0.0001)
Representative lesions of P-hyperintensity grade 0 without P-hyperplasia and P-hyperintensity grade 2 with P-hyperplasia. Representative lesions of P-hyperintensity grade 0 without P-hyperplasia (A, B) and P-hyperintensity grade 2 with P-hyperplasia (C, D). A This lesion has no P-hyperintensity on HB phase of EOB-MRI. B GS staining shows no hepatocytes with high GS expression indicating P-hyperplasia. (Original magnification ×20). C This lesion was evaluated as P-hyperintensity grade 2. D GS staining shows definite peri-tumoral hepatocytes with higher GS expression (P-hyperplasia). (Original magnification ×20)
P-hyperintensity grade 3 shows P-hyperplasia with over-expression of OATP1B3. A HB phase shows peri-tumoral hyperintensity around almost the whole boundary. This lesion was evaluated as P-hyperintensity grade 3. B GS staining shows definite P-hyperplasia. (Original magnification ×100). C In HE staining, P-hyperplasia can be distinguished from the background liver (Original magnification ×100). D High magnification of HE staining shows hyperplastic hepatocytes with no atypia at P-hyperplasia site. (Original magnification ×200) E Hepatocytes with GS expression indicating P-hyperplasia demonstrated definite membranous expression of OATP1B3 (uptake transporter of EOB) in all P-hyperplasia areas. Zonation of OATP1B3 expression was lost at P-hyperplasia sites. (Original magnification ×400) F In non-peri-tumoral liver parenchyma, OATP1B3 are expressed predominantly in the centrilobular hepatocytes (zone 3). (Original magnification ×400)
Correlation between the grade of P-hyperintensity and imaging and pathological features
The correlation between the presence of P-hyperintensity (grade 0 or grades 1–3) and imaging and pathological features was also analyzed (Table 4). There were no significant differences in the following factors including sex, age, size, hypovascular nodule on arterial phase, degree of corona enhancement, presence or absence of fibrous capsule, histology, macroscopic classification, and background liver fibrosis and inflammation. With regard to microvascular invasion in P-hyperintensity-positive HCCs (grades 1–3), the incidence of microscopic portal vein invasion, microscopic hepatic venous invasion, and microscopic vascular invasion (portal or hepatic venous) was seen in 20/43 (46.5%), 11/43 (25.6%), and 23/43 (53.3%), respectively. There was no significant difference in microscopic portal vein invasion (p = 0.4662), and microscopic vascular invasion (portal or hepatic venous) (p = 0.1828) among P-hyperintensity-positive (grade 1–3) and P-hyperintensity-negative (grade 0) HCCs. On the other hand, microscopic hepatic venous invasion was significantly more often seen in P-hyperintensity-positive group (grades 1–3) (p = 0.0017).
In addition, in this study peri-tumoral hypointensity was observed in 27 HCCs. In HCCs with peri-tumoral hypointensity, microscopic portal vein invasion, microscopic hepatic venous invasion and microscopic vascular invasion (portal or hepatic venous) were seen in 18/27 HCCs (66.7%), 8/27 (29.6%), and 20/27 HCCs (74.1%), respectively. And there was a significant difference in microscopic portal vein invasion (p = 0.0034), microscopic hepatic venous invasion (p = 0.0130) and microscopic vascular invasion (portal or hepatic venous) (p = 0.0007) between peri-tumoral hypointensity-positive HCCs (n = 27) and peri-tumoral hypointensity-negative HCCs (n = 50).
Between the two readers, the evaluation of the degree of corona enhancement differed for 5 HCCs (6.5%) and of hypovascularity in the arterial phase for 1 HCC (1.3%). Regarding inter-reader reproducibility, the weighted kappa value of the degree of corona enhancement and kappa value of hypovascularity were 0.909 and 0.902, respectively.
Discussion
In this study, there was a significant positive correlation between the presence of P-hyperplasia with over-expression of GS and the grade of P-hyperintensity. In addition, P-hyperplasia sites showed definite OATP1B3 (uptake transporter of Gd-EOB-DTPA) expression. Therefore, P-hyperintensity on the HB phase of EOB-MRI can be considered to reflect P-hyperplasia in the majority of lesions. The sensitivity and specificity of the P-hyperintensity predicting the presence of P-hyperplasia was 90.6% and 67.4%. We think that the reason for the relatively low specificity of this sign was due to the ambiguity of the finding of the grade 1.
As mentioned in the introduction, OATP1B3 and GS gene in peri-central hepatocytes are speculated to be regulated by Wnt/beta-catenin signaling [17,18,19,20]. In addition, in HCC and HCA a close relationship between OATP1B3 expression and Wnt/beta-catenin signaling including GS has been reported [12, 13, 21,22,23,24]. Therefore, GS might be closely related to OATP1B3 expression. In fact, OATP1B3 was well expressed at all P-hyperplasia sites with over-expression of GS in this study. The present result showing that p-hyperplasia with marked expression of GS has definite OATP1B3 expression can be explained by these molecular mechanisms. This might contribute to the hyperintensity seen on the HB phase of EOB-MRI.
As mentioned above, P-hyperplasia was diagnosed if there was a rim of hyperplastic hepatocytes of at least 0.5 mm thickness with plate architecture (including hepatocyte rosettes or thickened plates), delimited by a region of parenchymal atrophy with GS expression. In this study, P-hyperplasia was defined only by the presence of peri-tumoral hepatocytes rim with high GS expression. This is one of the important limitations of this study, and further pathological definition of this phenomenon is needed.
In this study, P-hyperintensity grade 1 had a lower incidence (55.2%) of histological P-hyperplasia compared with P-hyperintensity grade 2 nodules (90%) and 3 nodules (100%). This might be attributable to a misreading of artifacts or adjacent fine bile ducts as P-hyperintensity. Moreover, we did not evaluate the entire circumference histologically in this study, with this possibly being another cause of the lower incidence of histological P-hyperplasia noted in P-hyperintensity grade 1. In addition, fibrous capsule is thought to be the other possible cause of P-hyperintensity due to the retention of contrast media; however, in this study, the presence of fibrous capsule did not correlate with P-hyperintensity. Therefore, fibrous capsule may not be the main cause of P-hyperintensity. And also, the background fibrosis and inflammation also did not correlate with P-hyperintensity.
The pathogenesis of P-hyperplasia remains unclear. The following three possible causes have been considered: (1) perfusion abnormalities (due to vascular invasion), (2) regenerative changes in the liver cells induced by tumor compression, and (3) association with drainage blood flow. As for (1), Arnason et al. [25] suggested that P-hyperplasia is a hyperplastic response to increased arterial blood flow in the peri-tumoral parenchyma due to portal vein invasion. However, in this study, the frequency of portal vein invasion was not significantly increased in P-hyperintensity-positive (grade 1–3) group compared with negative of P-hyperintensity (grade 0). On the other hand, microscopic hepatic venous invasion was significantly more often seen in P-hyperintensity-positive (grade 1–3) group. Therefore, perfusion abnormalities due to microscopic hepatic venous invasion might be one cause of P-hyperintensity and P-hyperplasia. As for (2), GS has been also reported to be expressed in progenitor cells of the liver [33]. Therefore, regenerative changes may be one cause of P-hyperplasia. Concerning about (3), in HCC, corona enhancement due to drainage flow from the tumor into the surrounding liver is commonly observed. This drainage area does not receive portal blood flow and may be a cause of P-hyperplasia. However, in our study a close relationship was not noted between the degree of corona enhancement and P-hyperintensity grade. Therefore, drainage flow from the tumor may not directly contribute to the formation of P-hyperplasia.
In this study, although there was a statistically significant difference in microscopic hepatic vascular invasion between P-hyperintensity-positive group (grades 1–3) and P-hyperintensity-negative group (grade 0) (p = 0.0017), the incidence of microscopic hepatic venous invasion in P-hyperintensity-positive HCCs (grades 1–3) was relatively low (11/43; 25.6%). Therefore, we consider that the clinical impact of P-hyperintensity predicting microscopic hepatic venous invasion is thought to be limited.
On the other hand, previous study showed peri-tumoral hypointensity was correlated with higher incidence of microscopic vascular invasion. Kim et al. reported the incidence of microvascular invasion in HCC with peri-tumoral hypointensity was 88.5% [26]. In our study, HCC with peri-tumoral hypointensity was observed in 27/77 (35%) and the incidence of microvascular invasion (hepatic venous or portal venous) was 74.1%. Although the incidence rate of microvascular invasion in HCC with peri-tumoral hypointensity in our study was a little less frequent, a high incidence of microvascular invasion (hepatic venous or portal venous) was observed. In addition, there was a significant difference in microscopic portal vein invasion (p = 0.0034), microscopic hepatic venous invasion (p = 0.0130), and microscopic vascular invasion (portal or venous) (p = 0.0007) between peri-tumoral hypointensity-positive HCCs and peri-tumoral hypointensity-negative HCCs. On the other hand, there was no significant difference in microscopic portal venous invasion (p = 0.4662) and microvascular invasion (hepatic venous or portal venous) (p = 0.1828) between P-hyperintensity-positive (grade 1–3) and P-hyperintensity-negative group (grade 0). Therefore, we think that peri-tumoral hypointensity on HB phase is better imaging finding for prediction of microvascular invasion compared with P-hyperintensity.
The limitations of this study included the following. First, the histological definition of P-hyperplasia was not completely clear as mentioned above. Second, the entire circumference of the tumor was not evaluated in the histological examination, and therefore the histological analysis of P-hyperplasia was not robust. Third, the presence of artifacts and the limited resolution of the MR imaging may have modified to some degree the results of the image analyses.
In conclusion, peri-tumoral hyperintensity (P-hyperintensity) on HB phase of EOB-MRI in HCC may indicate peri-tumoral hyperplasia (P-hyperplasia) with GS and OATP1B3 expression and a higher incidence of microscopic hepatic venous invasion.
Abbreviations
- P-hyperintensity:
-
Peri-tumoral hyperintensity
- HCC:
-
Hepatocellular carcinoma
- HB:
-
Hepatobiliary
- EOB-MRI:
-
Gadoxetic acid-enhanced MRI
- P-hyperplasia:
-
Peri-tumoral hyperplasia
- GS:
-
Glutamine synthetase
- OATP:
-
Organic anion-transporting polypeptide
- MR:
-
Magnetic resonance
- HCA:
-
Hepatocellular adenoma
- HNF:
-
Hepatocyte nuclear factor
References
Schuhmann-Giampieri G, Schmitt-Willich H, Press W, et al. (1992) Preclinical evaluation of Gd-EOB-DTPA as a contrast agent in MR imaging of the hepatobiliary system. Radiology 183(1):59–64
Bluemke D, Sahani D, Amendola M, et al. (2005) Efficacy and safety of MR imaging with liver-specific contrast agent: U.S. multicenter phase III study. Radiology 237(1):89–98
Huppertz A, Balzer T, Blakeborough A, et al. (2004) Improved detection of focal liver lesions at MR imaging: multicenter comparison of gadoxetic acid-enhanced MR images with intraoperative findings. Radiology 230(1):266–275
Fu GL, Du Y, Zee CS, et al. (2012) Gadobenate dimeglumine-enhanced liver magnetic resonance imaging: value of hepatobiliary phase for the detection of focal liver lesions. J Comput Assist Tomogr 36(1):14–19. https://doi.org/10.1097/RCT.0b013e31823dc139
Purysko AS, Remer EM, Coppa CP, et al. (2012) Characteristics and distinguishing features of hepatocellular adenoma and focal nodular hyperplasia on gadoxetate disodium-enhanced MRI. AJR Am J Roentgenol 198(1):115–123. https://doi.org/10.2214/AJR.11.6836
Onishi H, Kim T, Imai Y, et al. (2012) Hypervascular hepatocellular carcinomas: detection with gadoxetate disodium-enhanced MR imaging and multiphasic multidetector CT. Eur Radiol 22(4):845–854. https://doi.org/10.1007/s00330-011-2316-y
Chung YE, Kim MJ, Kim YE, et al. (2013) Characterization of incidental liver lesions: comparison of multidetector CT versus Gd-EOB-DTPA-enhanced MR imaging. PloS ONE 8(6):e66141. https://doi.org/10.1371/journal.pone.0066141
Bottcher J, Hansch A, Pfeil A, et al. (2013) Detection and classification of different liver lesions: comparison of Gd-EOB-DTPA-enhanced MRI versus multiphasic spiral CT in a clinical single centre investigation. Eur J Radiol 82(11):1860–1869. https://doi.org/10.1016/j.ejrad.2013.06.013
Narita M, Hatano E, Arizono S, et al. (2009) Expression of OATP1B3 determines uptake of Gd-EOB-DTPA in hepatocellular carcinoma. J Gastroenterol 44(7):793–798
Kitao A, Zen Y, Matsui O, et al. (2010) Hepatocellular carcinoma: signal intensity at gadoxetic acid-enhanced MR Imaging–correlation with molecular transporters and histopathologic features. Radiology 256(3):817–826. https://doi.org/10.1148/radiol.10092214
Ba-Ssalamah A, Antunes C, Feier D, et al. (2015) Morphologic and molecular features of hepatocellular adenoma with gadoxetic acid-enhanced MR imaging. Radiology 277(1):104–113. https://doi.org/10.1148/radiol.2015142366
Fukusato T, Soejima Y, Kondo F, et al. (2015) Preserved or enhanced OATP1B3 expression in hepatocellular adenoma subtypes with nuclear accumulation of beta-catenin. Hepatol Res 45(10):E32–42. https://doi.org/10.1111/hepr.12453
Yoneda N, Matsui O, Kitao A, et al. (2012) Beta-catenin-activated hepatocellular adenoma showing hyperintensity on hepatobiliary-phase gadoxetic-enhanced magnetic resonance imaging and overexpression of OATP8. Jpn J Radiol 30(9):777–782. https://doi.org/10.1007/s11604-012-0115-2
Yoneda N, Matsui O, Kitao A, et al. (2012) Hepatocyte transporter expression in FNH and FNH-like nodule: correlation with signal intensity on gadoxetic acid enhanced magnetic resonance images. Jpn J Radiol 30(6):499–508. https://doi.org/10.1007/s11604-012-0085-4
Kitao A, Matsui O, Yoneda N, et al. (2011) The uptake transporter OATP8 expression decreases during multistep hepatocarcinogenesis: correlation with gadoxetic acid enhanced MR imaging. Eur Radiol 21(10):2056–2066. https://doi.org/10.1007/s00330-011-2165-8
Yoneda N, Matsui O, Kitao A, et al. (2016) Benign hepatocellular nodules: hepatobiliary phase of gadoxetic acid-enhanced MR imaging based on molecular background. Radiographics 36(7):2010–2027. https://doi.org/10.1148/rg.2016160037
Benhamouche S, Decaens T, Godard C, et al. (2006) Apc tumor suppressor gene is the “zonation-keeper” of mouse liver. Dev Cell 10(6):759–770. https://doi.org/10.1016/j.devcel.2006.03.015
Sekine S, Lan BY, Bedolli M, Feng S, Hebrok M (2006) Liver-specific loss of beta-catenin blocks glutamine synthesis pathway activity and cytochrome p450 expression in mice. Hepatology 43(4):817–825. https://doi.org/10.1002/hep.21131
Colletti M, Cicchini C, Conigliaro A, et al. (2009) Convergence of Wnt signaling on the HNF4alpha-driven transcription in controlling liver zonation. Gastroenterology 137(2):660–672. https://doi.org/10.1053/j.gastro.2009.05.038
Monga SP (2015) β-Catenin signaling and roles in liver homeostasis, injury, and tumorigenesis. Gastroenterology 148(7):1294–1310. https://doi.org/10.1053/j.gastro.2015.02.056
Sekine S, Ogawa R, Ojima H, Kanai Y (2011) Expression of SLCO1B3 is associated with intratumoral cholestasis and CTNNB1 mutations in hepatocellular carcinoma. Cancer Sci 102(9):1742–1747. https://doi.org/10.1111/j.1349-7006.2011.01990.x
Ueno A, Masugi Y, Yamazaki K, et al. (2014) OATP1B3 expression is strongly associated with Wnt/beta-catenin signalling and represents the transporter of gadoxetic acid in hepatocellular carcinoma. J Hepatol 61(5):1080–1087. https://doi.org/10.1016/j.jhep.2014.06.008
Kitao A, Matsui O, Yoneda N, et al. (2012) Hypervascular hepatocellular carcinoma: correlation between biologic features and signal intensity on gadoxetic acid-enhanced MR images. Radiology 265(3):780–789. https://doi.org/10.1148/radiol.12120226
Yamashita T, Kitao A, Matsui O, et al. (2014) Gd-EOB-DTPA-enhanced magnetic resonance imaging and alpha-fetoprotein predict prognosis of early-stage hepatocellular carcinoma. Hepatology 60(5):1674–1685. https://doi.org/10.1002/hep.27093
Arnason T, Fleming KE, Wanless IR (2013) Peritumoral hyperplasia of the liver: a response to portal vein invasion by hypervascular neoplasms. Histopathology 62(3):458–464. https://doi.org/10.1111/his.12032
Kim KA, Kim MJ, Jeon HM, et al. (2012) Prediction of microvascular invasion of hepatocellular carcinoma: usefulness of peritumoral hypointensity seen on gadoxetate disodium-enhanced hepatobiliary phase images. J Magn Reson Imaging 35(3):629–634. https://doi.org/10.1002/jmri.22876
Kitao A, Zen Y, Matsui O, Gabata T, Nakanuma Y (2009) Hepatocarcinogenesis: multistep changes of drainage vessels at CT during arterial portography and hepatic arteriography–radiologic-pathologic correlation. Radiology 252(2):605–614
International Consensus Group for Hepatocellular Neoplasia. The International Consensus Group for Hepatocellular N (2009) Pathologic diagnosis of early hepatocellular carcinoma: a report of the international consensus group for hepatocellular neoplasia. Hepatology 49(2):658–664. https://doi.org/10.1002/hep.22709
Bosman FT, World Health Organization., International Agency for Research on Cancer (2010) WHO classification of tumours of the digestive system. World Health Organization classification of tumours, vol. 3, 4th edn. Lyon: International Agency for Research on Cancer
Japan LCSGo (2010) General rules for the clinical and pathological study of primary liver cancer., 3rd ed. edn. Kanehara, Tokyo, Japan
Ichida F, Tsuji T, Omata M, et al. (1996) New Inuyama classification; New criteria for histological assessment of chronic hepatitis. Int Hepatol Commun 6(2):112–119. https://doi.org/10.1016/S0928-4346(96)00325-8
Vander Borght S, Libbrecht L, Blokzijl H, et al. (2005) Diagnostic and pathogenetic implications of the expression of hepatic transporters in focal lesions occurring in normal liver. J Pathol 207(4):471–482. https://doi.org/10.1002/path.1852
Fleming KE, Wanless IR (2013) Glutamine synthetase expression in activated hepatocyte progenitor cells and loss of hepatocellular expression in congestion and cirrhosis. Liver Int 33(4):525–534. https://doi.org/10.1111/liv.12099
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this study.
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Our institutional review board approved this retrospective study and waived the requirement for informed consent.
Rights and permissions
About this article
Cite this article
Yoneda, N., Matsui, O., Kitao, A. et al. Peri-tumoral hyperintensity on hepatobiliary phase of gadoxetic acid-enhanced MRI in hepatocellular carcinomas: correlation with peri-tumoral hyperplasia and its pathological features. Abdom Radiol 43, 2103–2112 (2018). https://doi.org/10.1007/s00261-017-1437-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-017-1437-4