Abstract
Background
Carbonic anhydrase IX (CA-IX) is a potential imaging biomarker of clear cell renal cell carcinoma (ccRCC). Here, we report the results of a phase II clinical trial of a small molecule radiotracer targeting CA-IX (18F-VM4-037) in ccRCC.
Methods
Between October 2012 and May 2013, 11 patients with kidney masses underwent 18F-VM4-037 PET/CT prior to surgery. Dynamic imaging was performed for the first 45 min post injection and whole-body imaging was obtained at 60 min post injection. Tumors were surgically excised or biopsied within 4 weeks of imaging.
Results
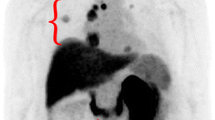
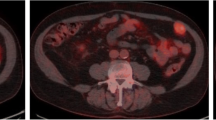
All patients tolerated the radiotracer well with no adverse events. Ten of the 11 patients had histologically confirmed malignancy. One patient had a Bosniak Type 3 cyst with no tumor found at surgery. Two patients had extrarenal disease and 9 had tumors only in the kidney. Primary ccRCC lesions were difficult to visualize on PET alone due to high uptake of the tracer in the adjacent normal kidney parenchyma, however when viewed in conjunction with CT, the tumors were easily localized. Metastatic lesions were clearly visible on PET. Mean SUV for primary kidney lesions was 2.55 in all patients; in patients with histologically confirmed ccRCC, the mean SUV was 3.16. The time-activity curves (TAC) are consistent with reversible ligand binding with peak activity concentration at 8 min post injection followed by washout. Distribution Volume Ratio (DVR) of the lesions was measured using the Logan graphical analysis method. The mean DVR value across the 9 kidney lesions was 5.2 ± 2.8, (range 0.68–10.34).
Conclusion
18F-VM4-037 is a well-tolerated PET agent that allows same day imaging of CA-IX expression. The agent demonstrated moderate signal uptake in primary tumors and excellent visualization of CA-IX positive metastases. While the evaluation of primary ccRCC lesions is challenging due to high background activity in the normal kidney parenchyma, 18F-VM4-037 may be most useful in the evaluation of metastatic ccRCC lesions.






Similar content being viewed by others
References
American Cancer Society (2014) Cancer facts & figures 2014. Atlanta: American Cancer Society
Potter CP, Harris AL (2003) Diagnostic, prognostic and therapeutic implications of carbonic anhydrases in cancer. Br J Cancer 89(1):2–7
Latif F, Tory K, Gnarra J, et al. (1993) Identification of the von Hippel–Lindau disease tumor suppressor gene. Science. 260(5112):1317–1320
Linehan WM, Walther MM, Zbar B (2003) The genetic basis of cancer of the kidney. J Urol 170(6 Pt 1):2163–2172
Rankin EB, Giaccia AJ (2008) The role of hypoxia-inducible factors in tumorigenesis. Cell Death Differ 15(4):678–685
Kaelin WG Jr (2008) The von Hippel–Lindau tumour suppressor protein: O2 sensing and cancer. Nat Rev Cancer 8(11):865–873
Kim WY, Kaelin WG (2004) Role of VHL gene mutation in human cancer. J Clin Oncol 22(24):4991–5004
Patard JJ, Fergelot P, Karakiewicz PI, et al. (2008) Low CAIX expression and absence of VHL gene mutation are associated with tumor aggressiveness and poor survival of clear cell renal cell carcinoma. Int J Cancer 123(2):395–400
Nyhan MJ, El Mashad SM, O’Donovan TR, et al. (2011) VHL genetic alteration in CCRCC does not determine de-regulation of HIF, CAIX, hnRNP A2/B1 and osteopontin. Cell Oncol 34(3):225–234
Leibovich BC, Sheinin Y, Lohse CM, et al. (2007) Carbonic anhydrase IX is not an independent predictor of outcome for patients with clear cell renal cell carcinoma. J Clin Oncol 25(30):4757–4764
Donato DP, Johnson MT, Yang XJ, Zynger DL (2011) Expression of carbonic anhydrase IX in genitourinary and adrenal tumours. Histopathology 59(6):1229–1239
Bui MH, Seligson D, Han KR, et al. (2003) Carbonic anhydrase IX is an independent predictor of survival in advanced renal clear cell carcinoma: implications for prognosis and therapy. Clin Cancer Res 9:802
Stillebroer AB, Boerman OC, Desar IM, et al. (2013) Phase 1 radioimmunotherapy study with lutetium 177-labeled anti-carbonic anhydrase IX monoclonal antibody girentuximab in patients with advanced renal cell carcinoma. Eur Urol 64:478
Divgi CR, Bander NH, Scott AM, et al. (1998) Phase I/II radioimmunotherapy trial with iodine-131-labeled monoclonal antibody G250 in metastatic renal cell carcinoma. Clin Cancer Res 4:2729
Divgi CR, Pandit-Taskar N, Jungbluth AA, et al. (2007) Preoperative characterisation of clear-cell renal carcinoma using iodine-124-labelled antibody chimeric G250 (124I-cG250) and PET in patients with renal masses: a phase I trial. Lancet Oncol 8:304
Muselaers CH, Boerman OC, Oosterwijk E, et al. (2013) Indium-111-labeled girentuximab immunoSPECT as a diagnostic tool in clear cell renal cell carcinoma. Eur Urol 63:1101
Oosterwijk E, Bander NH, Divgi CR, et al. (1993) Antibody localization in human renal cell carcinoma: a phase I study of monoclonal antibody G250. J Clin Oncol 11:738
Pryma DA, O’Donoghue JA, Humm JL, et al. (2011) Correlation of in vivo and in vitro measures of carbonic anhydrase IX antigen expression in renal masses using antibody 124I-cG250. J Nucl Med 52(4):535–540
Doss M, Kolb HC, Walsh JC, et al. (2014) Biodistribution and radiation dosimetry of the carbonic anhydrase IX imaging agent [(18) F]VM4-037 determined from PET/CT scans in healthy volunteers. Mol Imaging Biol 16(5):739–746
Surti S, Kuhn A, Werner ME, et al. (2007) Performance of Philips Gemini TF PET/CT scanner with special consideration for its time-of-flight imaging capabilities. J Nucl Med 48:471–480
Shuch B, Singer EA, Bratslavsky G (2012) The surgical approach to multifocal renal cancers: hereditary syndromes, ipsilateral multifocality, and bilateral tumors. Urol Clin North Am 39:133
Makis W, Ciarallo A, Rakheja R, et al. (2012) Spectrum of malignant renal and urinary bladder tumors on 18F-FDG PET/CT: a pictorial essay. Clin Imaging 36:660
Bouchelouche K, Choyke PL (2015) PET/computed tomography in renal, bladder, and testicular cancer. PET Clin 10(3):361–374
Acknowledgments
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. This research was supported [in part] by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. Special thanks to the Urologic Oncology Fellows of the Urologic Oncology Branch of the National Cancer Institute, NIH; Avi Rosenberg, MD, PhD, Laboratory of Pathology, National Cancer Institute, NIH; Hartmut Kolb from Siemens and PET/NET for supplying the agent.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Baris Turkbey and Adam R. Metwalli have contributed equally to the writing and preparation of this manuscript.
Rights and permissions
About this article
Cite this article
Turkbey, B., Lindenberg, M.L., Adler, S. et al. PET/CT imaging of renal cell carcinoma with 18F-VM4-037: a phase II pilot study. Abdom Radiol 41, 109–118 (2016). https://doi.org/10.1007/s00261-015-0599-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-015-0599-1