Abstract
Purpose
The purpose of this study was to evaluate whether affected bowel in Crohn’s disease patients can be identified by observing decreased peristalsis (frozen bowel sign) using cine balanced steady-state free precession (cine BSSFP) images.
Materials and methods
5 radiologists independently reviewed cine BSSFP sequences from randomized MR Enterography (MRE) exams for 30 normal and 30 Crohn’s disease patients, graded overall small bowel peristalsis from slowest to fastest, and graded peristalsis for the most abnormal small bowel segment. Sensitivity and specificity of the frozen bowel sign for diagnosing Crohn’s disease were calculated. T tests of the peristalsis difference between abnormal segments and overall small bowel were conducted.
Results
For 5 readers, the sensitivity and specificity of cine BSSFP of the frozen bowel sign for diagnosing Crohn’s disease ranged from 70% to 100% and 87% to 100%, respectively. There were significant differences in peristalsis between abnormal small bowel segments and the overall small bowel for Crohn’s patients, but not in the overall small bowel between normal-MRE patients and Crohn’s disease patients.
Conclusion
Abnormal Crohn’s small bowel segments have significantly decreased peristalsis compared to normal small bowel, which can be identified using cine BSSFP sequences as the frozen bowel sign.

Similar content being viewed by others
References
Booya F, Fletcher JG, Huprich JE, et al. (2006) Active crohn disease: CT findings and interobserver agreement for enteric phase CT enterography. Radiology 241(3):787–795.
Bodily KD, Fletcher JG, Solem CA, et al. (2006) Crohn disease: mural attenuation and thickness at contrast-enhanced CT enterography—correlation with endoscopic and histologic findings of inflammation. Radiology 238(2):505–516.
Choi D, Jin LS, Ah CY, et al. (2003) Bowel wall thickening in patients with Crohn’s disease: CT patterns and correlation with inflammatory activity. Clin Radiol 58(1):68–74.
Maglinte DDT, Gourtsoyiannis N, Rex D, et al. (2003) Classification of small bowel Crohn’s subtypes based on multimodality imaging. Radiol Clin North Am 41(2):285–304.
Florie J, Wasser MNJM, Arts-Cieslik K, et al. (2006) Dynamic contrast-enhanced MRI of the bowel wall for assessment of disease activity in Crohn’s disease. Am J Roentgenol 186(5):1384–1392.
Siddiki HA, Fidler JL, Fletcher JG, et al. (2009) Prospective comparison of state-of-the-art MR enterography and CT enterography in small-bowel Crohn’s disease. Am J Roentgenol 193(1):113–121.
Rimola J, Rodríguez S, García-Bosch O, et al. (2009) Magnetic resonance for assessment of disease activity and severity in ileocolonic Crohn’s disease. Gut 58(8):1113–1120.
Messaris E, Chandolias N, Grand D, et al. (2010) Role of magnetic resonance enterography in the management of Crohn disease. Arch Surg 145(5):471–475.
Punwani S, Rodriguez-Justo M, Bainbridge A, et al. (2009) Mural inflammation in crohn disease: location-matched histologic validation of MR imaging features. Radiology 252(3):712–720.
Sinha R, Rajiah P, Murphy P, et al. (2009) Utility of high-resolution MR imaging in demonstrating transmural pathologic changes in Crohn disease. Radiographics 29(6):1847–1867.
Steward MJ, Punwani S, Proctor I, et al. (2011) Non-perforating small bowel Crohn’s disease assessed by MRI enterography: Derivation and histopathological validation of an MR-based activity index. Eur J Radiol 81(9):2080–2088.
Ziech M, Bossuyt P, Laghi A, et al. (2011) Grading luminal Crohn’s disease: Which MRI features are considered as important? Eur J Radiol 81(4):e467–e472.
Ziech MLW, Bipat S, Roelofs JJTH, et al. (2011) Retrospective comparison of magnetic resonance imaging features and histopathology in Crohn’s disease patients. Eur J Radiol 80(3):e299–e305.
Grand DJ, Kampalath V, Harris A, et al. (2012) MR enterography correlates highly with colonoscopy and histology for both distal ileal and colonic Crohn’s disease in 310 patients. Eur J Radiol 81(5):e763–e769.
Sempere GAJ, Sanjuan VM, Chulia EM, et al. (2005) MRI evaluation of inflammatory activity in Crohn’s disease. Am J Roentgenol 184(6):1829–1835.
Oto A, Zhu F, Kulkarni K, et al. (2009) Evaluation of diffusion-weighted MR imaging for detection of bowel inflammation in patients with Crohn’s disease. Acad Radiol 16(5):597–603.
Oto A, Kayhan A, Williams JTB, et al. (2011) Active Crohn’s disease in the small bowel: evaluation by diffusion weighted imaging and quantitative dynamic contrast enhanced MR imaging. J Magn Reson Imaging 33(3):615–624.
Oussalah A, Laurent V, Bruot O, et al. (2010) Diffusion-weighted magnetic resonance without bowel preparation for detecting colonic inflammation in inflammatory bowel disease. Gut 59(8):1056–1065.
Kiryu S, Dodanuki K, Takao H, et al. (2009) Free-breathing diffusion-weighted imaging for the assessment of inflammatory activity in Crohn’s disease. J Magn Reson Imaging 29(4):880–886.
Ghobrial PM, Neuberger I, Guglielmo FF, et al. (2014) Cine MR enterography grading of small bowel peristalsis. evaluation of the antiperistaltic effectiveness of sublingual hyoscyamine sulfate. Acad Radiol 21(1):86–91.
Wakamiya M, Furukawa A, Kanasaki S, et al. (2011) Assessment of small bowel motility function With cine-MRI using balanced steady-state free precession sequence. J Magn Reson Imaging 33(5):1235–1240.
Patak MA, Froehlich JM, Von Weymarn C, et al. (2007) Non-invasive measurement of small-bowel motility by MRI after abdominal surgery. Gut 56(7):1023–1025.
Froehlich JM, Patak MA, von Weymarn C, et al. (2005) Small bowel motility assessment with magnetic resonance imaging. J Magn Reson Imaging 21(4):370–375.
Odille F, Menys A, Ahmed A et al. (2012) Quantitative assessment of small bowel motility by nonrigid registration of dynamic MR images. Magn Reson Med 68(3):783–793.
Heye T, Stein D, Antolovic D, et al. (2012) Evaluation of bowel peristalsis by dynamic cine MRI: Detection of relevant functional disturbances—initial experience. J Magn Reson Imaging 35(4):859–867.
Gutzeit A, Binkert CA, Koh DM et al. (2012) Evaluation of the anti-peristaltic effect of glucagon and hyoscine on the small bowel: comparison of intravenous and intramuscular drug administration. Eur Radiol 22(6):1186–1194.
Kitazume Y, Satoh S, Hosoi H, et al. (2007) Cine magnetic resonance imaging evaluation of peristalsis of small bowel with longitudinal ulcer in Crohn disease: preliminary results. J Comput Assist Tomogr 31(6):876–883.
Menys A, Atkinson D, Odille F et al. (2012) Quantified terminal ileal motility during MR enterography as a potential biomarker of Crohn’s disease activity: a preliminary study. Eur Radiol 22(11):2494–2501.
Bickelhaupt S, Pazahr S, Chuck N et al. Crohn’s disease: small bowel motility impairment correlates with inflammatory-related markers C-reactive protein and calprotectin. Neurogastroenterol Motil 2013;25(6):467–e363.
Girometti R, Zuiani C, Toso F, et al. (2008) MRI scoring system including dynamic motility evaluation in assessing the activity of Crohn’s disease of the terminal ileum. Acad Radiol 15(2):153–164.
Froehlich JM, Waldherr C, Stoupis C, et al. (2010) MR motility imaging in Crohn’s disease improves lesion detection compared with standard MR imaging. Eur Radiol 20(8):1945–1951.
Froehlich JM, Daenzer M, von Weymarn C, et al. (2009) Aperistaltic effect of hyoscine N-butylbromide versus glucagon on the small bowel assessed by magnetic resonance imaging. Eur Radiol 19(6):1387–1393.
Shrout PE, Fleiss JL (1979) Intraclass correlations: uses in assessing rater reliability. Psychol Bull 86(2):420.
Koh D, Miao Y, Chinn R, et al. (2001) MR imaging evaluation of the activity of Crohn’s disease. Am J Roentgenol 177(6):1325–1332
Grand DJ, Beland MD, Machan JT et al. Detection of Crohn’s disease: Comparison of CT and MR enterography without anti-peristaltic agents performed on the same day. Eur J Radiol 2011;81(8):1735–1741.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure S1. Cine balanced steady state free precession (cine BSSFP) series showing the 5 progressive grades of peristalsis ranging from Peristalsis Grade 1 (slowest) to Peristalsis Grade 5 (fastest). (S1A = Peristalsis Grade 1, S1B = Peristalsis Grade 2, S1C = Peristalsis Grade 3, S1D = Peristalsis Grade 4, S1E = Peristalsis Grade 5)
Supplementary Figure S1A. (Multimedia Video)—Cine balanced fast field echo (BFFE) series (TR 3.1 ms, TE 1.5 ms,matrix 160 x 160, flip angle 45°, thickness 8 mm) showing Peristalsis Grade 1 (MP4 7225 kb)
261_2014_258_MOESM2_ESM.mp4
Supplementary Figure S1B. (Multimedia Video)—Cine balanced fast field echo (BFFE) series (TR 3.2 ms, TE 1.6 ms,matrix 160 x 160, flip angle 45°, thickness 8 mm) showing Peristalsis Grade 2 (MP4 7129 kb)
261_2014_258_MOESM3_ESM.mp4
Supplementary Figure S1C. (Multimedia Video)—Cine balanced fast field echo (BFFE) series (TR 3.3 ms, TE 1.6 ms,matrix 160 x 160, flip angle 45°, thickness 8 mm) showing Peristalsis Grade 3 (MP4 6839 kb)
261_2014_258_MOESM4_ESM.mp4
Supplementary Figure S1D. (Multimedia Video)—Cine balanced fast field echo (BFFE) series (TR 3.2 ms, TE 1.6 ms,matrix 160 x 160, flip angle 45°, thickness 8 mm) showing Peristalsis Grade 4 (MP4 8658 kb)
261_2014_258_MOESM5_ESM.mp4
Supplementary Figure S1E. (Multimedia Video)—Cine true fast imaging with steady-state precession (TRUE FISP) series (TR 4.9 ms, TE 2.2 ms, matrix 320 x 240, flip angle 65°, thickness 8 mm) showing Peristalsis Grade 5 (MP4 9605 kb)
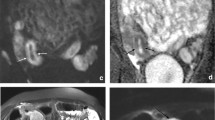
Supplementary Figure S2. Active Crohn’s disease of the terminal ileum in a 33 year old woman
Supplementary Figure S2A. (Multimedia video)—Cine balanced fast field echo (BFFE) series (TR 3.2 ms, TE 1.6 ms, matrix 176 × 178, flip angle 45°, thickness 8 mm) showing decreased peristalsis of the abnormal terminal ileum (arrow) compared to the remainder of the small bowel (MP4 1153 kb)
261_2014_258_MOESM7_ESM.tif
Supplementary Figure S2B. Coronal (TR ∞, TE 200 ms, matrix 308 × 268, thickness 6 mm) T2-weighted single-shot fast spin echo (FSE) image showing mild terminal ileum wall thickening and wall edema (blue arrows) (TIF 1264 kb)
261_2014_258_MOESM8_ESM.tif
Supplementary Figure S2C. 3-D dynamic post-contrast coronal T1 W high-resolution isotropic volume examination (THRIVE) image (TR 4.1 ms, TE 2 ms, matrix 168 × 167, thickness 5 mm) showing increased late arterial transmural enhancement predominantly in the mesenteric wall (arrows) with enlarged adjacent lymph nodes (arrowhead) consistent with active Crohn’s disease (TIF 882 kb)
Supplementary Figure S3. Active Crohn’s disease of the terminal ileum in a 25 year old woman
Supplementary Figure S3A. (Multimedia video)—Cine balanced fast field echo (BFFE) series (TR 3.2 ms, TE 1.6 ms, matrix 160 × 160, flip angle 45°, thickness 8 mm) showing decreased peristalsis of the abnormal terminal ileum (arrows) compared to the remainder of the small bowel (MP4 3047 kb)
261_2014_258_MOESM10_ESM.tif
Supplementary Figure S3B. Coronal T2-weighted single-shot fast spin echo (FSE) images (TR ∞, TE 80 ms, matrix 256 × 154, thickness 6 mm) showing mild terminal ileum wall thickening (arrows) (TIF 925 kb)
261_2014_258_MOESM11_ESM.tif
Supplementary Figure S3C. 3-D dynamic (S3C) and delayed post-contrast (S3D) coronal T1W high-resolution isotropic volume examination (THRIVE) images (TR 4.2 ms, TE 2.1 ms, matrix 256 × 163, thickness 5 mm) showing mucosal fold thickening with increased mucosal and transmural late arterial enhancement (S3C) and delayed enhancement (S3D) with ulceration along the lateral wall (arrows) consistent with active Crohn’s disease (TIF 891 kb)
261_2014_258_MOESM12_ESM.tif
Supplementary Figure S3D. 3-D dynamic (S3C) and delayed post-contrast (S3D) coronal T1W high-resolution isotropic volume examination (THRIVE) images (TR 4.2 ms, TE 2.1 ms, matrix 256 × 163, thickness 5 mm) showing mucosal fold thickening with increased mucosal and transmural late arterial enhancement (S3C) and delayed enhancement (S3D) with ulceration along the lateral wall (arrows) consistent with active Crohn’s disease (TIF 835 kb)
Supplementary Figure S4. Active inflammatory and chronic Crohn’s disease of the terminal ileum with adjacent phlegmon in a 21 year old woman
Supplementary Figure S4A. (Multimedia video)—Cine fast imaging employing steady-state acquisition (FIESTA) series (TR 3.8 ms, TE 1.7 ms, matrix 224 × 288, flip angle 70°, thickness 8 mm) showing decreased peristalsis of the abnormal terminal ileum (arrow) compared to the remainder of the small bowel, and adjacent phlegmon (arrowhead). There is upstream small bowel dilatation (MP4 2458 kb)
261_2014_258_MOESM14_ESM.tif
Supplementary Figure S4B. Coronal T2-weighted single-shot fast spin echo (FSE) image (TR ∞, TE 181 ms, matrix 256 × 224, thickness 5 mm) showing wall thickening of the terminal ileum (arrows) and adjacent phlegmon (arrowheads) (TIF 947 kb)
261_2014_258_MOESM15_ESM.tif
Supplementary Figure S4C. Dynamic post-contrast coronal liver acquisition with volume acceleration (LAVA) images (TR 4 ms, TE 1.76 ms, matrix 320 × 224, thickness 4.4 mm) showing terminal ileum wall thickening and mucosal hyperenhancement (arrows) (TIF 584 kb)
261_2014_258_MOESM16_ESM.tif
Supplementary Figure S4D. Dynamic post-contrast coronal liver acquisition with volume acceleration (LAVA) images (TR 4 ms, TE 1.76 ms, matrix 320 × 224, thickness 4.4 mm) showing terminal ileum wall thickening and mucosal hyperenhancement (arrows) and enhancing adjacent phlegmon (arrowheads) (TIF 860 kb)
Supplementary Figure S5. Active inflammatory and chronic Crohn’s disease of the mid ileum in a 66 year old man
Supplementary Figure S5A. (Multimedia video)—Cine balanced fast field echo (BFFE) series (TR 3.2 ms, TE 1.6 ms, matrix 160 × 160, flip angle 45°, thickness 8 mm) showing decreased peristalsis of the abnormal mid ileum (arrows) compared to the remainder of the small bowel with mild upstream small bowel dilatation (MP4 2969 kb)
261_2014_258_MOESM18_ESM.tif
Supplementary Figure S5B. Coronal T2-weighted single-shot image (TR ∞, TE 80 ms, matrix 256 × 154, thickness 6 mm) showing wall thickening, wall edema, and areas of narrowing with skip lesions in the mid ileum (arrows) (TIF 1041 kb)
261_2014_258_MOESM19_ESM.tif
Supplementary Figure S5C. 3-D dynamic post-contrast coronal T1W high-resolution isotropic volume examination (THRIVE) image (TR 4.16 ms, TE 2.1 ms, matrix 256 × 163, thickness 5 mm) showing wall thickening with mucosal and serosal hyperenhancement causing a stratified enhancement pattern (arrows) (TIF 999 kb)
261_2014_258_MOESM20_ESM.tif
Supplementary Figure S5D. 3-D delayed post-contrast coronal THRIVE image (TR 4.16 ms, TE 2.1 ms, matrix 256 × 163, thickness 5 mm) showing more homogeneous wall enhancement (TIF 989 kb)
Rights and permissions
About this article
Cite this article
Guglielmo, F.F., Mitchell, D.G., O’Kane, P.L. et al. Identifying decreased peristalsis of abnormal small bowel segments in Crohn’s disease using cine MR enterography: the frozen bowel sign. Abdom Imaging 40, 1150–1156 (2015). https://doi.org/10.1007/s00261-014-0258-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-014-0258-y