Abstract
Purpose
Lutetium-177 [177Lu]Lu-PSMA-617 radioligand therapy (RLT) represents a significant advancement for metastatic castration-resistant prostate cancer (mCRPC), demonstrating improvements in radiographic progression free survival (rPFS) and overall survival (OS) with a low rate of associated side effects. Currently, most post-therapy SPECT/CT is conducted at 24 h after infusion. This study examines the clinical utility of a next-generation multi-detector Cadmium-Zinc-Telluride (CZT) SPECT/CT system (StarGuide) in same-day post-infusion assessment and early treatment response to [177Lu]Lu-PSMA-617.
Methods
In this retrospective study, 68 men with progressive mCRPC treated with [177Lu]Lu-PSMA-617 at our center from June 2022 to June 2023 were evaluated. Digital whole-body SPECT/CT imaging was performed after [177Lu]Lu-PSMA-617infusion (mean ± SD: 1.8 ± 0.6 h, range 1.1–4.9 h). Quantitative analysis of [177Lu]Lu-PSMA-617 positive lesions was performed in patients who underwent at least 2 post-therapy SPECT/CT, using liver parenchyma uptake as reference. Metrics including [177Lu]Lu-PSMA-617 positive total tumor volume (Lu-TTV), SUVmax and SUVmean were calculated. These quantitative metrics on post-infusion SPECT/CT images after cycles 1, 2 and 3 were correlated with overall survival (OS), prostate specific antigen-progression free survival (PSA-PFS) as defined by prostate cancer working group 3 (PCWG3), and PSA decrease over 50% (PSA50) response rates.
Results
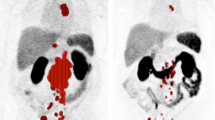
56 patients (means age 76.2 ± 8.1 years, range: 60–93) who underwent at least 2 post-therapy SPECT/CT were included in the image analysis. The whole-body SPECT/CT scans (~ 12 min per scan) were well tolerated, with 221 same-day scans performed (89%). At a median of 10-months follow-up, 33 (58.9%) patients achieved PSA50 after [177Lu]Lu-PSMA-617 treatment and median PSA-PFS was 5.0 months (range: 1.0–15 months) while median OS was not reached. Quantitative analysis of SPECT/CT images showed that 37 patients (66%) had > 30% reduction in Lu-TTV, associated with significantly improved overall survival (median not reached vs. 6 months, P = 0.008) and PSA-PFS (median 6 months vs. 1 months, P < 0.001). However, changes in SUVmax or SUVmean did not correlate with PSA-PFS or OS.
Conclusion
We successfully implemented same-day post-therapy SPECT/CT after [177Lu]Lu-PSMA-617 infusions. Quantitation of 1–2 h post-therapy SPECT/CT images is a promising method for assessing treatment response. However, the approach is currently limited by its suboptimal detection of small tumor lesions and the necessity of incorporating a third-cycle SPECT/CT to mitigate the effects of any potential treatment-related flare-up. Further investigation in a larger patient cohort and prospective validation is essential to confirm these findings and to explore the role of SPECT/CT as a potential adjunct to PSMA PET/CT in managing mCRPC.




Similar content being viewed by others
Data availability
Yes.
Code availability
Not applicable.
References
Sartor O, de Bono J, Chi KN, Fizazi K, Herrmann K, Rahbar K, et al. Lutetium-177-PSMA-617 for metastatic castration-resistant prostate Cancer. N Engl J Med. 2021;385:1091–103.
Hofman MS, Emmett L, Sandhu S, Iravani A, Joshua AM, Goh JC, et al. [(177)Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. Lancet. 2021;397:797–804.
Hofman MS, Violet J, Hicks RJ, Ferdinandus J, Thang SP, Akhurst T, et al. [(177)Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study. Lancet Oncol. 2018;19:825–33.
Sadaghiani MS, Sheikhbahaei S, Werner RA, Pienta KJ, Pomper MG, Solnes LB, et al. A systematic review and Meta-analysis of the effectiveness and toxicities of Lutetium-177-labeled prostate-specific membrane Antigen-targeted Radioligand Therapy in Metastatic Castration-resistant prostate Cancer. Eur Urol. 2021;80:82–94.
Gafita A, Rauscher I, Weber M, Hadaschik B, Wang H, Armstrong WR, et al. Novel Framework for treatment response evaluation using PSMA PET/CT in patients with metastatic castration-resistant prostate Cancer (RECIP 1.0): an International Multicenter Study. J Nucl Med. 2022;63:1651–8.
Fanti S, Goffin K, Hadaschik BA, Herrmann K, Maurer T, MacLennan S, et al. Consensus statements on PSMA PET/CT response assessment criteria in prostate cancer. Eur J Nucl Med Mol Imaging. 2021;48:469–76.
Gafita A, Calais J, Grogan TR, Hadaschik B, Wang H, Weber M, et al. Nomograms to predict outcomes after (177)Lu-PSMA therapy in men with metastatic castration-resistant prostate cancer: an international, multicentre, retrospective study. Lancet Oncol. 2021;22:1115–25.
Buteau JP, Martin AJ, Emmett L, Iravani A, Sandhu S, Joshua AM, et al. PSMA and FDG-PET as predictive and prognostic biomarkers in patients given [(177)Lu]Lu-PSMA-617 versus cabazitaxel for metastatic castration-resistant prostate cancer (TheraP): a biomarker analysis from a randomised, open-label, phase 2 trial. Lancet Oncol. 2022;23:1389–97.
Grubmuller B, Senn D, Kramer G, Baltzer P, D’Andrea D, Grubmuller KH, et al. Response assessment using (68)Ga-PSMA ligand PET in patients undergoing (177)Lu-PSMA radioligand therapy for metastatic castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging. 2019;46:1063–72.
John N, Pathmanandavel S, Crumbaker M, Counter W, Ho B, Yam AO, et al. (177)Lu-PSMA SPECT quantitation at 6 weeks (dose 2) predicts short progression-free survival for patients undergoing (177)Lu-PSMA-I&T Therapy. J Nucl Med. 2023;64:410–5.
Pathmanandavel S, Crumbaker M, Ho B, Yam AO, Wilson P, Niman R et al. Evaluation of (177)Lu-PSMA SPECT quantitation as a response biomarker within a prospective (177)Lu-PSMA-617 and NOX66 combination trial (LuPIN). J Nucl Med. 2022.
Kratochwil C, Fendler WP, Eiber M, Hofman MS, Emmett L, Calais J, et al. Joint EANM/SNMMI procedure guideline for the use of (177)Lu-labeled PSMA-targeted radioligand-therapy ((177)Lu-PSMA-RLT). Eur J Nucl Med Mol Imaging. 2023;50:2830–45.
Gupta A, Eisenhauer EA, Booth CM. The Time toxicity of Cancer Treatment. J Clin Oncol. 2022;40:1611–5.
Song H, Ferri V, Duan H, Aparici CM, Davidzon G, Franc BL, et al. SPECT at the speed of PET: a feasibility study of CZT-based whole-body SPECT/CT in the post (177)Lu-DOTATATE and (177)Lu-PSMA617 setting. Eur J Nucl Med Mol Imaging. 2023;50:2250–7.
LE ROUZIC GZ, Rani. First performance measurements of a new multi-detector CZT-Based SPECT/CT system: GE StarGuide. J Nucl Med. 2021;62:1125.
Scher HI, Morris MJ, Stadler WM, Higano C, Basch E, Fizazi K, et al. Trial Design and objectives for castration-resistant prostate Cancer: updated recommendations from the prostate Cancer clinical trials Working Group 3. J Clin Oncol. 2016;34:1402–18.
Gafita A, DJAILEB L, Rauscher I, Fendler W, Hadaschik B, Herrmann K, et al. Practical RECIP: visual assessment of response evaluation Criteria in PSMA-PET/CT in metastatic castration-resistant prostate cancer. J Nucl Med. 2023;64:P1619–P.
Sartor O. Phase 3 trial of [177Lu]Lu-PSMA-617 in taxane-naive patients with metastatic castration-resistant prostate cancer (PSMAfore). ESMO Congress. 2023.
Seifert R, Kessel K, Schlack K, Weber M, Herrmann K, Spanke M, et al. PSMA PET total tumor volume predicts outcome of patients with advanced prostate cancer receiving [(177)Lu]Lu-PSMA-617 radioligand therapy in a bicentric analysis. Eur J Nucl Med Mol Imaging. 2021;48:1200–10.
Pathmanandavel S, Crumbaker M, Ho B, Yam AO, Wilson P, Niman R, et al. Evaluation of (177)Lu-PSMA-617 SPECT/CT quantitation as a response Biomarker within a prospective (177)Lu-PSMA-617 and NOX66 combination trial (LuPIN). J Nucl Med. 2023;64:221–6.
Neubauer MC, Nicolas GP, Bauman A, Fani M, Nitzsche E, Afshar-Oromieh A, et al. Early response monitoring during [(177)Lu]Lu-PSMA I&T therapy with quantitated SPECT/CT predicts overall survival of mCRPC patients: subgroup analysis of a swiss-wide prospective registry study. Eur J Nucl Med Mol Imaging. 2024;51:1185–93.
Ritt P. Recent developments in SPECT/CT. Semin Nucl Med. 2022;52:276–85.
Sjogreen Gleisner K, Chouin N, Gabina PM, Cicone F, Gnesin S, Stokke C, et al. EANM dosimetry committee recommendations for dosimetry of 177Lu-labelled somatostatin-receptor- and PSMA-targeting ligands. Eur J Nucl Med Mol Imaging. 2022;49:1778–809.
Brosch-Lenz J, Delker A, Volter F, Unterrainer LM, Kaiser L, Bartenstein P, et al. Toward single-time-point image-based dosimetry of (177)Lu-PSMA-617 therapy. J Nucl Med. 2023;64:767–74.
Jackson PA, Hofman MS, Hicks RJ, Scalzo M, Violet J. Radiation Dosimetry in (177)Lu-PSMA-617 therapy using a single posttreatment SPECT/CT scan: a novel methodology to Generate Time- and tissue-specific dose factors. J Nucl Med. 2020;61:1030–6.
Derlin T, Widjaja L, Werner RA, Bengel FM. (177)Lu-PSMA for Extended Treatment of Metastatic Castration-resistant prostate Cancer. J Nucl Med. 2022.
Mader N, Nguyen Ngoc C, Kirkgoze B, Baumgarten J, Groener D, Klimek K, et al. Extended therapy with [(177)Lu]Lu-PSMA-617 in responding patients with high-volume metastatic castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging. 2023;50:1811–21.
Wahl RL, Sgouros G, Iravani A, Jacene H, Pryma D, Saboury B, et al. Normal-tissue tolerance to Radiopharmaceutical Therapies, the knowns and the unknowns. J Nucl Med. 2021;62:S23–35.
Violet J, Jackson P, Ferdinandus J, Sandhu S, Akhurst T, Iravani A, et al. Dosimetry of (177)Lu-PSMA-617 in metastatic castration-resistant prostate Cancer: correlations between Pretherapeutic Imaging and whole-body tumor dosimetry with treatment outcomes. J Nucl Med. 2019;60:517–23.
Capala J, Graves SA, Scott A, Sgouros G, James SS, Zanzonico P, et al. Dosimetry for Radiopharmaceutical Therapy: current practices and Commercial resources. J Nucl Med. 2021;62:S3–11.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
HS content planning, data analyses, manuscript writing and editing; MIL data analyses, manuscript editing; VF data analyses, manuscript editing; HD data analyses, manuscript editing; CMA manuscript editing; GD manuscript editing; BLF manuscript editing; FM manuscript editing; JS manuscript editing; CPM manuscript editing; ACF manuscript editing; SS manuscript editing; ARK manuscript editing; SS manuscript editing; AI content planning, data analyses, manuscript editing.
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in our study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Stanford University Institutional Review Board approved this retrospective data analysis.
Conflicts of interest
Andrei Iagaru is an Associate Editor at the European Journal of Nuclear Medicine and Molecular Imaging. Hong Song, Maria Isabel Leonio, Valentina Ferri, Heying Duan, Carina Mari Aparici, Guido Davidzon, Benjamin L. Franc, Farshad Moradi, Jagruti Shah, Colin P Bergstrom, Alice C Fan, Sumit Shah, Ali Raza Khaki and Sandy Srinivas declare that they have no conflict of interest or competing interests.
Consent for publication
Waived by the Stanford University Institutional Review Board for this retrospective data analysis.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Song, H., Leonio, M.I., Ferri, V. et al. Same-day post-therapy imaging with a new generation whole-body digital SPECT/CT in assessing treatment response to [177Lu]Lu-PSMA-617 in metastatic castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging (2024). https://doi.org/10.1007/s00259-024-06718-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00259-024-06718-6