Abstract
Purpose
Histone deacetylase 6 (HDAC6) has emerged as a therapeutic target for neurodegenerative diseases such as Alzheimer’s disease. Noninvasive imaging of HDAC6 in the brain by positron emission tomography (PET) would accelerate research into its roles in these diseases. We recently discovered an 18F-labeled derivative of the selective HDAC6 inhibitor SW-100 ([18F]FSW-100) as a potential candidate for brain HDAC6 radioligand. As a mandatory step prior to clinical translation, we performed preclinical validation of [18F]FSW-100.
Methods
Process validation of [18F]FSW-100 radiosynthesis for clinical use and assessment of preclinical toxicity and radiation dosimetry estimated from mouse distribution data were performed. In vitro selectivity of FSW-100 for 28 common receptors in the brain and HDAC isoforms was characterized. [18F]FSW-100 PET imaging was performed in non-human primates in a conscious state to estimate the feasibility of HDAC6 imaging in humans.
Results
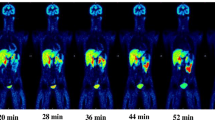
Three consecutive validation runs of the automated radiosynthesis gave [18F]FSW-100 injections with radiochemical yields of 12%, and the injections conformed to specified quality control criteria for batch release. No acute toxicity was observed for non-radiolabeled FSW-100 or radioactivity decayed [18F]FSW-100 injection, and the former was negative in the Ames test. The whole-body effective dose estimated from biodistribution in mice was within the range of that of previously reported 18F-radioligands in humans. In vitro selectivity against common receptors and other HDAC isoforms was confirmed. [18F]FSW-100 demonstrated good penetration in monkey brain, and in vivo blocking studies suggested that the uptake was specific.
Conclusion
These results support the clinical utility of [18F]FSW-100 for in vivo imaging of HDAC6 in the brain.




Similar content being viewed by others
Data availability
The data obtained in the current study are available from the corresponding author on reasonable request.
References
Barneda-Zahonero B, Parra M. Histone deacetylases and cancer. Mol Oncol. 2012;6:579–89. https://doi.org/10.1016/j.molonc.2012.07.003.
Batchu SN, Brijmohan AS, Advani A. The therapeutic hope for HDAC6 inhibitors in malignancy and chronic disease. Clin Sci (Lond). 2016;130:987–1003. https://doi.org/10.1042/CS20160084.
Bertos NR, Gilquin B, Chan GK, Yen TJ, Khochbin S, Yang XJ. Role of the tetradecapeptide repeat domain of human histone deacetylase 6 in cytoplasmic retention. J Biol Chem. 2004;279:48246–54. https://doi.org/10.1074/jbc.M408583200.
Li Y, Shin D, Kwon SH. Histone deacetylase 6 plays a role as a distinct regulator of diverse cellular processes. FEBS J. 2013;280:775–93. https://doi.org/10.1111/febs.12079.
Cohen TJ, Guo JL, Hurtado DE, Kwong LK, Mills IP, Trojanowski JQ, et al. The acetylation of tau inhibits its function and promotes pathological tau aggregation. Nat Commun. 2011;2:252. https://doi.org/10.1038/ncomms1255.
Miki Y, Mori F, Tanji K, Kakita A, Takahashi H, Wakabayashi K. Accumulation of histone deacetylase 6, an aggresome-related protein, is specific to Lewy bodies and glial cytoplasmic inclusions. Neuropathology. 2011;31:561–8. https://doi.org/10.1111/j.1440-1789.2011.01200.x.
Kawaguchi Y, Kovacs JJ, McLaurin A, Vance JM, Ito A, Yao TP. The deacetylase HDAC6 regulates aggresome formation and cell viability in response to misfolded protein stress. Cell. 2003;115:727–38.
Simoes-Pires C, Zwick V, Nurisso A, Schenker E, Carrupt PA, Cuendet M. HDAC6 as a target for neurodegenerative diseases: what makes it different from the other HDACs? Mol Neurodegener. 2013;8:7. https://doi.org/10.1186/1750-1326-8-7.
Ding H, Dolan PJ, Johnson GV. Histone deacetylase 6 interacts with the microtubule-associated protein tau. J Neurochem. 2008;106:2119–30. https://doi.org/10.1111/j.1471-4159.2008.05564.x.
Odagiri S, Tanji K, Mori F, Miki Y, Kakita A, Takahashi H, et al. Brain expression level and activity of HDAC6 protein in neurodegenerative dementia. Biochem Biophys Res Commun. 2013;430:394–9. https://doi.org/10.1016/j.bbrc.2012.11.034.
Guo W, Naujock M, Fumagalli L, Vandoorne T, Baatsen P, Boon R, et al. HDAC6 inhibition reverses axonal transport defects in motor neurons derived from FUS-ALS patients. Nat Commun. 2017;8:861. https://doi.org/10.1038/s41467-017-00911-y.
Onishi T, Maeda R, Terada M, Sato S, Fujii T, Ito M, et al. A novel orally active HDAC6 inhibitor T-518 shows a therapeutic potential for Alzheimer’s disease and tauopathy in mice. Sci Rep. 2021;11:15423. https://doi.org/10.1038/s41598-021-94923-w.
Tago T, Toyohara J. Advances in the development of PET ligands targeting histone deacetylases for the assessment of neurodegenerative diseases. Molecules. 2018;23. https://doi.org/10.3390/molecules23020300.
Schroeder FA, Wang C, Van de Bittner GC, Neelamegam R, Takakura WR, Karunakaran A, et al. PET imaging demonstrates histone deacetylase target engagement and clarifies brain penetrance of known and novel small molecule inhibitors in rat. ACS Chem Neurosci. 2014;5:1055–62. https://doi.org/10.1021/cn500162j.
Del Rosso G, Carlomagno Y, Todd TW, Jones CY, Prudencio M, Daughrity LM, et al. HDAC6 interacts with poly (GA) and modulates its accumulation in c9FTD/ALS. Front Cell Dev Biol. 2021;9: 809942. https://doi.org/10.3389/fcell.2021.809942.
Strebl MG, Campbell AJ, Zhao WN, Schroeder FA, Riley MM, Chindavong PS, et al. HDAC6 brain mapping with [18F]bavarostat enabled by a Ru-mediated deoxyfluorination. ACS Cent Sci. 2017;3:1006–14. https://doi.org/10.1021/acscentsci.7b00274.
Koole M, Van Weehaeghe D, Serdons K, Herbots M, Cawthorne C, Celen S, et al. Clinical validation of the novel HDAC6 radiotracer [18F]EKZ-001 in the human brain. Eur J Nucl Med Mol Imaging. 2021;48:596–611. https://doi.org/10.1007/s00259-020-04891-y.
Bai P, Mondal P, Bagdasarian FA, Rani N, Liu Y, Gomm A, et al. Development of a potential PET probe for HDAC6 imaging in Alzheimer’s disease. Acta Pharm Sin B. 2022;12:3891–904. https://doi.org/10.1016/j.apsb.2022.05.017.
Kozikowski AP, Shen S, Pardo M, Tavares MT, Szarics D, Benoy V, et al. Brain penetrable histone deacetylase 6 inhibitor SW-100 ameliorates memory and learning impairments in a mouse model of fragile X syndrome. ACS Chem Neurosci. 2019;10:1679–95. https://doi.org/10.1021/acschemneuro.8b00600.
Tago T, Toyohara J, Ishii K. Preclinical evaluation of an 18F-labeled SW-100 derivative for PET imaging of histone deacetylase 6 in the brain. ACS Chem Neurosci. 2021;12:746–55. https://doi.org/10.1021/acschemneuro.0c00774.
Onoe H, Inoue O, Suzuki K, Tsukada H, Itoh T, Mataga N, et al. Ketamine increases the striatal N-[11C]methylspiperone binding in vivo: positron emission tomography study using conscious rhesus monkey. Brain Res. 1994;663:191–8. https://doi.org/10.1016/0006-8993(94)91263-7.
Logan J, Fowler JS, Volkow ND, Wolf AP, Dewey SL, Schlyer DJ, et al. Graphical analysis of reversible radioligand binding from time-activity measurements applied to [N-11C-methyl]-(-)-cocaine PET studies in human subjects. J Cereb Blood Flow Metab. 1990;10:740–7. https://doi.org/10.1038/jcbfm.1990.127.
Varga J, Szabo Z. Modified regression model for the Logan plot. J Cereb Blood Flow Metab. 2002;22:240–4. https://doi.org/10.1097/00004647-200202000-00012.
Lassen NA, Bartenstein PA, Lammertsma AA, Prevett MC, Turton DR, Luthra SK, et al. Benzodiazepine receptor quantification in vivo in humans using [11C]flumazenil and PET: application of the steady-state principle. J Cereb Blood Flow Metab. 1995;15:152–65. https://doi.org/10.1038/jcbfm.1995.17.
Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–61. https://doi.org/10.1096/fj.07-9574LSF.
Jochems J, Boulden J, Lee BG, Blendy JA, Jarpe M, Mazitschek R, et al. Antidepressant-like properties of novel HDAC6-selective inhibitors with improved brain bioavailability. Neuropsychopharmacology. 2014;39:389–400. https://doi.org/10.1038/npp.2013.207.
Toyohara J, Nishino K, Sakai M, Tago T, Oda T. Automated production of [18F]MK-6240 on CFN-MPS200. Appl Radiat Isot. 2020;168: 109468. https://doi.org/10.1016/j.apradiso.2020.109468.
Tetrabutylammonium in radiopharmaceutical preparations. European Pharmacopoeia. 10.5 ed: European Directorate for the Quality of Medicines & HealthCare. 2021, pp.5661.
Bogni A, Laera L, Cucchi C, Seregni E, Pascali C. Tetrabutylammonium HPLC analysis: shortcomings in the Ph. Eur method J Labelled Comp Radiopharm. 2020;63:203–8. https://doi.org/10.1002/jlcr.3822.
ICH Guideline Q3D(R2) Guideline for elemental impurities. https://database.ich.org/sites/default/files/Q3D-R2_Guideline_Step4_2022_0308.pdf. Accessed 20 Sep 2023.
Shen S, Kozikowski AP. Why hydroxamates may not be the best histone deacetylase inhibitors–what some may have forgotten or would rather forget? ChemMedChem. 2016;11:15–21. https://doi.org/10.1002/cmdc.201500486.
Shen S, Picci C, Ustinova K, Benoy V, Kutil Z, Zhang G, et al. Tetrahydroquinoline-capped histone deacetylase 6 inhibitor SW-101 ameliorates pathological phenotypes in a charcot-marie-tooth type 2A mouse model. J Med Chem. 2021;64:4810–40. https://doi.org/10.1021/acs.jmedchem.0c02210.
Koziorowski J, Behe M, Decristoforo C, Ballinger J, Elsinga P, Ferrari V, et al. Position paper on requirements for toxicological studies in the specific case of radiopharmaceuticals. EJNMMI Radiopharm Chem. 2017;1:1. https://doi.org/10.1186/s41181-016-0004-6.
Dietz KC, Casaccia P. HDAC inhibitors and neurodegeneration: at the edge between protection and damage. Pharmacol Res. 2010;62:11–7. https://doi.org/10.1016/j.phrs.2010.01.011.
Anderson KW, Chen J, Wang M, Mast N, Pikuleva IA, Turko IV. Quantification of histone deacetylase isoforms in human frontal cortex, human retina, and mouse brain. PLoS ONE. 2015;10: e0126592. https://doi.org/10.1371/journal.pone.0126592.
Guidance for Industry and Researchers. The radioactive drug research committee: human research without an investigational new drug application: US Food and Drug Administration. 2010.
Zanotti-Fregonara P, Lammertsma AA, Innis RB. 11C dosimetry scans should be abandoned. J Nucl Med. 2021;62:158–9. https://doi.org/10.2967/jnumed.120.257402.
Radiological Protection in Biomedical Research. ICRP Publication 62. Ann ICRP. 1992;22.
Zanotti-Fregonara P, Lammertsma AA, Innis RB. Suggested pathway to assess radiation safety of 18F-labeled PET tracers for first-in-human studies. Eur J Nucl Med Mol Imaging. 2013;40:1781–3. https://doi.org/10.1007/s00259-013-2512-x.
Celen S, Rokka J, Gilbert TM, Koole M, Vermeulen I, Serdons K, et al. Translation of HDAC6 PET imaging using [18F]EKZ-001-cGMP production and measurement of HDAC6 target occupancy in nonhuman primates. ACS Chem Neurosci. 2020;11:1093–101. https://doi.org/10.1021/acschemneuro.0c00074.
Lechner S, Malgapo MIP, Gratz C, Steimbach RR, Baron A, Ruther P, et al. Target deconvolution of HDAC pharmacopoeia reveals MBLAC2 as common off-target. Nat Chem Biol. 2022;18:812–20. https://doi.org/10.1038/s41589-022-01015-5.
Acknowledgements
The authors would like to thank Mr. Kosuke Nishino and Mr. Masanari Sakai (SHI Accelerator Service Ltd, Tokyo, Japan) for technical support with the cyclotron operation and radiosynthesis.
Funding
This work was supported by Grants-in-Aid for Young Scientists (No. 20K16778) and Scientific Research (C) (No. 22K07682) from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Contributions
T.T., J.T., and K.I. designed the study. T.T. performed the radiosynthesis studies. T.T. and M.S. performed the dosimetry study. T.T., M.S., M.K., S.Y., and J.T. contributed to the NHP PET study. T.T. and J.T. wrote the manuscript. K.I. commented on the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Studies using rodents were approved by the Animal Care and Use Committee of TMIG (Approval Nos. 21023, 22011, and 22012) and Bozo Research Center (Approval Nos. G220322, G220323). Studies using monkeys were carried out in accordance with the recommendations of the US NIH and approved by the Ethics Committee of the Central Research Laboratory, Hamamatsu Photonics (Approval No. HPK-2022–09) and the Animal Care and Use Committee of TMIG (Approval No. 22009).
Competing interests
M.K. and S.Y. are employed by Hamamatsu Photonics. The other authors declare no competing financial interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tago, T., Sakata, M., Kanazawa, M. et al. Preclinical validation of a novel brain-penetrant PET ligand for visualization of histone deacetylase 6: a potential imaging target for neurodegenerative diseases. Eur J Nucl Med Mol Imaging (2024). https://doi.org/10.1007/s00259-024-06666-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00259-024-06666-1