Abstract
Purpose
There are image interpretation criteria to standardize reporting prostate-specific membrane antigen (PSMA)-targeted positron emission tomography (PET). As up to 10% of prostate cancer (PC) do not express PSMA, other targets such as gastrin-releasing peptide receptor (GRPR) are evaluated. Research on GRPR-targeted imaging has been slowly increasing in usage at staging and biochemical recurrence (BCR) of PC. We therefore propose a modification of the Prostate Cancer Molecular Imaging Standardized Evaluation (PROMISE) criteria (mPROMISE) for GRPR-targeted PET.
Methods
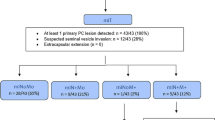
[68 Ga]Ga-RM2 PET data from initially prospective studies performed at our institution were retrospectively reviewed: 44 patients were imaged for staging and 100 patients for BCR PC. Two nuclear medicine physicians independently evaluated PET according to the mPROMISE criteria. A third expert reader served as standard reference. Interreader reliability was computed for GRPR expression, prostate bed (T), lymph node (N), skeleton (Mb), organ (Mc) metastases, and final judgment of the scan.
Results
The interrater reliability for GRPR PET at staging was moderate for GRPR expression (0.59; 95% confidence interval [CI] 0.40, 0.78), substantial for T-stage (0.78; 95% CI 0.63, 0.94), and almost perfect for N-stage (0.97; 95% CI 0.92, 1.00) and final judgment (0.92; 95% CI 0.82, 1.00). The interreader agreement at BCR showed substantial agreement for GRPR expression (0.70; 95% CI 0.59, 0.81) and final judgment (0.65; 95% CI 0.53, 0.78), while almost perfect agreement was seen across the major categories (T, N, Mb, Mc). Acceptable performance of the mPROMISE criteria was found for all subsets when compared to the standard reference.
Conclusion
Interpreting GRPR-targeted PET using the mPROMISE criteria showed its reliability with substantial or almost perfect interrater agreement across all major categories. The proposed modification of the PROMISE criteria will aid clinicians in decreasing the level of uncertainty, and clinical trials to achieve uniform evaluation, reporting, and comparability of GRPR-targeted PET.
Trial registration
Clinicaltrials.gov Identifier: NCT03113617 and NCT02624518.





Similar content being viewed by others
Data availability
Yes.
Code availability
Yes.
References
Fanti S, Minozzi S, Morigi JJ, Giesel F, Ceci F, Uprimny C, et al. Development of standardized image interpretation for 68Ga-PSMA PET/CT to detect prostate cancer recurrent lesions. Eur J Nucl Med Mol Imaging. 2017;44:1622–35. https://doi.org/10.1007/s00259-017-3725-1.
Rowe SP, Pienta KJ, Pomper MG, Gorin MA. PSMA-RADS version 1.0: a step towards standardizing the interpretation and reporting of PSMA-targeted PET imaging studies. Eur Urol. 2018;73:485–7. https://doi.org/10.1016/j.eururo.2017.10.027.
Eiber M, Herrmann K, Calais J, Hadaschik B, Giesel FL, Hartenbach M, et al. Prostate cancer molecular imaging standardized evaluation (PROMISE): proposed miTNM classification for the interpretation of PSMA-ligand PET/CT. J Nucl Med. 2018;59:469–78. https://doi.org/10.2967/jnumed.117.198119.
Seifert R, Emmett L, Rowe SP, Herrmann K, Hadaschik B, Calais J, et al. Second version of the prostate cancer molecular imaging standardized evaluation framework including response evaluation for clinical trials (PROMISE V2). Eur Urol. 2023. https://doi.org/10.1016/j.eururo.2023.02.002.
Emmett L, Papa N, Buteau J, Ho B, Liu V, Roberts M, et al. The PRIMARY score: using intraprostatic (68)Ga-PSMA PET/CT patterns to optimize prostate cancer diagnosis. J Nucl Med. 2022;63:1644–50. https://doi.org/10.2967/jnumed.121.263448.
Maurer T, Gschwend JE, Rauscher I, Souvatzoglou M, Haller B, Weirich G, et al. Diagnostic efficacy of (68)gallium-PSMA positron emission tomography compared to conventional imaging for lymph node staging of 130 consecutive patients with intermediate to high risk prostate cancer. J Urol. 2016;195:1436–43. https://doi.org/10.1016/j.juro.2015.12.025.
Budaus L, Leyh-Bannurah SR, Salomon G, Michl U, Heinzer H, Huland H, et al. Initial experience of (68)Ga-PSMA PET/CT imaging in high-risk prostate cancer patients prior to radical prostatectomy. Eur Urol. 2016;69:393–6. https://doi.org/10.1016/j.eururo.2015.06.010.
Duan H, Baratto L, Fan RE, Soerensen SJC, Liang T, Chung BI, et al. Correlation of (68)Ga-RM2 PET with postsurgery histopathology findings in patients with newly diagnosed intermediate- or high-risk prostate cancer. J Nucl Med. 2022;63:1829–35. https://doi.org/10.2967/jnumed.122.263971.
Baratto L, Song H, Duan H, Hatami N, Bagshaw H, Buyyounouski M, et al. PSMA- and GRPR-targeted PET: results from 50 patients with biochemically recurrent prostate cancer. J Nucl Med. 2021. https://doi.org/10.2967/jnumed.120.259630.
Mapelli P, Ghezzo S, Samanes Gajate AM, Preza E, Palmisano A, Cucchiara V, et al. (68)Ga-PSMA and (68)Ga-DOTA-RM2 PET/MRI in recurrent prostate cancer: diagnostic performance and association with clinical and histopathological data. Cancers (Basel). 2022;14. https://doi.org/10.3390/cancers14020334.
Minamimoto R, Sonni I, Hancock S, Vasanawala S, Loening A, Gambhir SS, et al. Prospective evaluation of (68)Ga-RM2 PET/MRI in patients with biochemical recurrence of prostate cancer and negative findings on conventional imaging. J Nucl Med. 2018;59:803–8. https://doi.org/10.2967/jnumed.117.197624.
Touijer KA, Michaud L, Alvarez HAV, Gopalan A, Kossatz S, Gonen M, et al. Prospective study of the radiolabeled GRPR antagonist BAY86-7548 for positron emission tomography/computed tomography imaging of newly diagnosed prostate cancer. Eur Urol Oncol. 2019;2:166–73. https://doi.org/10.1016/j.euo.2018.08.011.
Mapelli P, Ghezzo S, Samanes Gajate AM, Preza E, Brembilla G, Cucchiara V, et al. Preliminary results of an ongoing prospective clinical trial on the use of (68)Ga-PSMA and (68)Ga-DOTA-RM2 PET/MRI in staging of high-risk prostate cancer patients. Diagnostics (Basel). 2021;11. https://doi.org/10.3390/diagnostics11112068.
Baratto L, Duan H, Laudicella R, Toriihara A, Hatami N, Ferri V, et al. Physiological (68)Ga-RM2 uptake in patients with biochemically recurrent prostate cancer: an atlas of semi-quantitative measurements. Eur J Nucl Med Mol Imaging. 2020;47:115–22. https://doi.org/10.1007/s00259-019-04503-4.
Cookson MS, Aus G, Burnett AL, Canby-Hagino ED, D’Amico AV, Dmochowski RR, et al. Variation in the definition of biochemical recurrence in patients treated for localized prostate cancer: the American Urological Association Prostate Guidelines for Localized Prostate Cancer Update Panel report and recommendations for a standard in the reporting of surgical outcomes. J Urol. 2007;177:540–5. https://doi.org/10.1016/j.juro.2006.10.097.
Roach M 3rd, Hanks G, Thames H Jr, Schellhammer P, Shipley WU, Sokol GH, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65:965–74. https://doi.org/10.1016/j.ijrobp.2006.04.029.
Toriihara A, Nobashi T, Baratto L, Duan H, Moradi F, Park S, et al. Comparison of 3 interpretation criteria for (68)Ga-PSMA11 PET based on inter- and intrareader agreement. J Nucl Med. 2020;61:533–9. https://doi.org/10.2967/jnumed.119.232504.
Demirci E, Akyel R, Caner B, Alan-Selcuk N, Guven-Mese S, Ocak M, et al. Interobserver and intraobserver agreement on prostate-specific membrane antigen PET/CT images according to the miTNM and PSMA-RADS criteria. Nucl Med Commun. 2020;41:759–67. https://doi.org/10.1097/MNM.0000000000001219.
Duan H, Ghanouni P, Daniel B, Rosenberg J, Thong A, Kunder C, et al. A pilot study of (68)Ga-PSMA11 and (68)Ga-RM2 PET/MRI for biopsy guidance in patients with suspected prostate cancer. J Nucl Med. 2022. https://doi.org/10.2967/jnumed.122.264448.
Duan H, Ghanouni P, Daniel B, Rosenberg J, Davidzon GA, Mari Aparici C, et al. A pilot study of (68)Ga-PSMA11 and (68)Ga-RM2 PET/MRI for evaluation of prostate cancer response to high intensity focused ultrasound (HIFU) therapy. J Nucl Med. 2022. https://doi.org/10.2967/jnumed.122.264783.
Beer M, Montani M, Gerhardt J, Wild PJ, Hany TF, Hermanns T, et al. Profiling gastrin-releasing peptide receptor in prostate tissues: clinical implications and molecular correlates. Prostate. 2012;72:318–25. https://doi.org/10.1002/pros.21434.
Korner M, Waser B, Rehmann R, Reubi JC. Early over-expression of GRP receptors in prostatic carcinogenesis. Prostate. 2014;74:217–24. https://doi.org/10.1002/pros.22743.
Fendler WP, Calais J, Allen-Auerbach M, Bluemel C, Eberhardt N, Emmett L, et al. (68)Ga-PSMA-11 PET/CT interobserver agreement for prostate cancer assessments: an international multicenter prospective study. J Nucl Med. 2017;58:1617–23. https://doi.org/10.2967/jnumed.117.190827.
Nickols N, Anand A, Johnsson K, Brynolfsson J, Borreli P, Parikh N, et al. aPROMISE: a novel automated PROMISE platform to standardize evaluation of tumor burden in (18)F-DCFPyL images of veterans with prostate cancer. J Nucl Med. 2022;63:233–9. https://doi.org/10.2967/jnumed.120.261863.
Author information
Authors and Affiliations
Contributions
HD: content planning, data analyses, manuscript writing, and editing; GAD: data analyses and manuscript editing; FM: data analyses and manuscript editing; TL: data analyses and manuscript editing; HS: manuscript editing; AI: content planning, data analyses, and manuscript editing.
Corresponding author
Ethics declarations
Ethics approval
Yes.
Consent to participate
Yes.
Consent for publication
Yes.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Duan, H., Davidzon, G.A., Moradi, F. et al. Modified PROMISE criteria for standardized interpretation of gastrin-releasing peptide receptor (GRPR)-targeted PET. Eur J Nucl Med Mol Imaging 50, 4087–4095 (2023). https://doi.org/10.1007/s00259-023-06385-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-023-06385-z