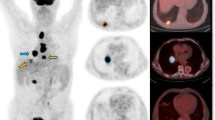
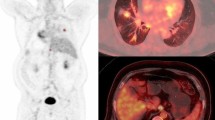
Abstract
Purpose
This study aimed to investigate the predictive value of metabolic features in response to induction immuno-chemotherapy in patients with locally advanced non-small cell cancer (LA-NSCLC), using ultra-high sensitivity dynamic total body [18F]FDG PET/CT.
Methods
The study analyzed LA-NSCLC patients who received two cycles of induction immuno-chemotherapy and underwent a 60-min dynamic total body [18F]FDG PET/CT scan before treatment. The primary tumors (PTs) were manually delineated, and their metabolic features, including the Patlak-Ki, Patlak-Intercept, maximum SUV (SUVmax), metabolic tumor volume (MTV) and total lesion glycolysis (TLG) were evaluated. The overall response rate (ORR) to induction immuno-chemotherapy was evaluated according to RECIST 1.1 criteria. The Patlak-Ki of PTs was calculated from the 20–60 min frames using the Patlak graphical analysis. The best feature was selected using Laplacian feature importance scores, and an unsupervised K-Means method was applied to cluster patients. ROC curve was used to examine the effect of selected metabolic feature in predicting tumor response to treatment. The targeted next generation sequencing on 1021 genes was conducted. The expressions of CD68, CD86, CD163, CD206, CD33, CD34, Ki67 and VEGFA were assayed through immunohistochemistry. The independent samples t test and the Mann–Whitney U test were applied in the intergroup comparison. Statistical significance was considered at P < 0.05.
Results
Thirty-seven LA-NSCLC patients were analyzed between September 2020 and November 2021. All patients received two cycles of induction chemotherapy combined with Nivolumab/ Camrelizumab. The Laplacian scores showed that the Patlak-Ki of PTs had the highest importance for patient clustering, and the unsupervised K-Means derived decision boundary of Patlak-Ki was 2.779 ml/min/100 g. Patients were categorized into two groups based on their Patlak-Ki values: high FDG Patlak-Ki (H-FDG-Ki, Patlak-Ki > 2.779 ml/min/100 g) group (n = 23) and low FDG Patlak-Ki (L-FDG-Ki, Patlak-Ki ≤ 2.779 ml/min/100 g) group (n = 14). The ORR to induction immuno-chemotherapy was 67.6% (25/37) in the whole cohort, with 87% (20/23) in H-FDG-Ki group and 35.7% (5/14) in L-FDG-Ki group (P = 0.001). The sensitivity and specificity of Patlak-Ki in predicting the treatment response were 80% and 75%, respectively [AUC = 0.775 (95%CI 0.605–0.945)]. The expression of CD3+/CD8+ T cells and CD86+/CD163+/CD206+ macrophages were higher in the H-FDG-Ki group, while Ki67, CD33+ myeloid cells, CD34+ micro-vessel density (MVD) and tumor mutation burden (TMB) were comparable between the two groups.
Conclusions
The total body [18F]FDG PET/CT scanner performed a dynamic acquisition of the entire body and clustered LA-NSCLC patients into H-FDG-Ki and L-FDG-Ki groups based on the Patlak-Ki. Patients with H-FDG-Ki demonstrated better response to induction immuno-chemotherapy and higher levels of immune cell infiltration in the PTs compared to those with L-FDG-Ki. Further studies with a larger patient cohort are required to validate these findings.










Similar content being viewed by others
Data availability
The datasets are available from the corresponding authors on reasonable request.
References
Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373:123–35.
Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med. 2016;375:1823–33.
Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–65.
Tseng JS, Yang TY, Wu CY, et al. Characteristics and Predictive Value of PD-L1 Status in Real-World Non-Small Cell Lung Cancer Patients. J Immunother. 2018;41:292–9.
Aguilar EJ, Ricciuti B, Gainor JF, et al. Outcomes to first-line pembrolizumab in patients with non-small-cell lung cancer and very high PD-L1 expression. Ann Oncol. 2019;30:1653–9.
Hong L, Negrao MV, Dibaj SS, et al. Programmed Death-Ligand 1 Heterogeneity and Its Impact on Benefit From Immune Checkpoint Inhibitors in NSCLC. J Thorac Oncol. 2020;15:1449–59.
Borghaei H, Langer CJ, Paz-Ares L, et al. Pembrolizumab plus chemotherapy versus chemotherapy alone in patients with advanced non-small cell lung cancer without tumor PD-L1 expression: A pooled analysis of 3 randomized controlled trials. Cancer. 2020;126:4867–77.
Provencio M, Nadal E, Insa A, et al. Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. 2020;21:1413–22.
Forde PM, Spicer J, Lu S, et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N Engl J Med. 2022;386:1973–85.
Paz-Ares L, Spira A, Raben D, et al. Outcomes with durvalumab by tumour PD-L1 expression in unresectable, stage III non-small-cell lung cancer in the PACIFIC trial. Ann Oncol. 2020;31:798–806.
Sorace AG, Elkassem AA, Galgano SJ, et al. Imaging for Response Assessment in Cancer Clinical Trials. Semin Nucl Med. 2020;50:488–504.
Wang D, Qiu B, He H, et al. Tumor response evaluation by combined modalities of chest magnetic resonance imaging and computed tomography in locally advanced non-small cell lung cancer after concurrent chemoradiotherapy. Radiother Oncol. 2022;168:211–20.
Vriens D, Disselhorst JA, Oyen WJ, et al. Quantitative assessment of heterogeneity in tumor metabolism using FDG-PET. Int J Radiat Oncol Biol Phys. 2012;82:e725-731.
Katiyar P, Divine MR, Kohlhofer U, et al. Spectral Clustering Predicts Tumor Tissue Heterogeneity Using Dynamic (18)F-FDG PET: A Complement to the Standard Compartmental Modeling Approach. J Nucl Med. 2017;58:651–7.
Hyun SH, Kim HS, Choi SH, et al. Intratumoral heterogeneity of (18)F-FDG uptake predicts survival in patients with pancreatic ductal adenocarcinoma. Eur J Nucl Med Mol Imaging. 2016;43:1461–8.
Sanli Y, Leake J, Odu A, et al. Tumor heterogeneity on FDG PET/CT and immunotherapy: an imaging biomarker for predicting treatment response in patients with metastatic melanoma. AJR Am J Roentgenol. 2019;212(6):1318–26.
Hashimoto K, Kaira K, Yamaguchi O, et al. Potential of FDG-PET as prognostic significance after anti-PD-1 antibody against patients with previously treated non-small cell lung cancer. J Clin Med. 2020;9(3):725.
Kaira K, Higuchi T, Naruse I, et al. Metabolic activity by (18)F-FDG-PET/CT is predictive of early response after nivolumab in previously treated NSCLC. Eur J Nucl Med Mol Imaging. 2018;45:56–66.
Jreige M, Letovanec I, Chaba K, et al. (18)F-FDG PET metabolic-to-morphological volume ratio predicts PD-L1 tumour expression and response to PD-1 blockade in non-small-cell lung cancer. Eur J Nucl Med Mol Imaging. 2019;46:1859–68.
Zhang X, Xie Z, Berg E, et al. Total-Body Dynamic Reconstruction and Parametric Imaging on the uEXPLORER. J Nucl Med. 2020;61:285–91.
Wang D, Zhang X, Liu H, et al. Assessing dynamic metabolic heterogeneity in non-small cell lung cancer patients via ultra-high sensitivity total-body [(18)F]FDG PET/CT imaging: quantitative analysis of [(18)F]FDG uptake in primary tumors and metastatic lymph nodes. Eur J Nucl Med Mol Imaging. 2022;49:4692–704.
Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Gandhi L, Rodriguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med. 2018;378:2078–92.
Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N Engl J Med. 2018;379:2040–51.
Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med. 2018;378:2288–301.
Carbone DP, Reck M, Paz-Ares L, et al. First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer. N Engl J Med. 2017;376:2415–26.
Paz-Ares L, Langer CJ, Novello S, et al. LBA80 - Pembrolizumab (pembro) plus platinum-based chemotherapy (chemo) for metastatic NSCLC: Tissue TMB (tTMB) and outcomes in KEYNOTE-021, 189, and 407. Ann Oncol. 2019;30:v917–8.
Vekens K, Everaert H, Neyns B, et al. The Value of (18)F-FDG PET/CT in Predicting the Response to PD-1 Blocking Immunotherapy in Advanced NSCLC Patients with High-Level PD-L1 Expression. Clin Lung Cancer. 2021;22:432–40.
Takada K, Toyokawa G, Yoneshima Y, et al. (18)F-FDG uptake in PET/CT is a potential predictive biomarker of response to anti-PD-1 antibody therapy in non-small cell lung cancer. Sci Rep. 2019;9:13362.
Liu G, Xu H, Hu P, et al. Kinetic metrics of (18)F-FDG in normal human organs identified by systematic dynamic total-body positron emission tomography. Eur J Nucl Med Mol Imaging. 2021;48:2363–72.
Liu G, Hu P, Yu H, et al. Ultra-low-activity total-body dynamic PET imaging allows equal performance to full-activity PET imaging for investigating kinetic metrics of (18)F-FDG in healthy volunteers. Eur J Nucl Med Mol Imaging. 2021;48:2373–83.
Sari H, Mingels C, Alberts I, et al. First results on kinetic modelling and parametric imaging of dynamic (18)F-FDG datasets from a long axial FOV PET scanner in oncological patients. Eur J Nucl Med Mol Imaging. 2022;49:1997–2009.
Strauss LG, Klippel S, Pan L, et al. Assessment of quantitative FDG PET data in primary colorectal tumours: which parameters are important with respect to tumour detection? Eur J Nucl Med Mol Imaging. 2007;34:868–77.
Epelbaum R, Frenkel A, Haddad R, et al. Tumor aggressiveness and patient outcome in cancer of the pancreas assessed by dynamic 18F-FDG PET/CT. J Nucl Med. 2013;54:12–8.
Yang Z, Zan Y, Zheng X, et al. Dynamic FDG-PET Imaging to Differentiate Malignancies from Inflammation in Subcutaneous and In Situ Mouse Model for Non-Small Cell Lung Carcinoma (NSCLC). PLoS ONE. 2015;10: e0139089.
Patlak CS, Blasberg RG, Fenstermacher JD. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J Cereb Blood Flow Metab. 1983;3:1–7.
Visser EP, Philippens ME, Kienhorst L, et al. Comparison of tumor volumes derived from glucose metabolic rate maps and SUV maps in dynamic 18F-FDG PET. J Nucl Med. 2008;49:892–8.
Magri A, Krol A, Lee W, et al. A new method to determine probability of malignancy using dynamic breast F-18-FDG PET studies. J Nucl Med. 2009;50:1445–1445.
Sugawara Y, Zasadny KR, Grossman HB, et al. Germ cell tumor: differentiation of viable tumor, mature teratoma, and necrotic tissue with FDG PET and kinetic modeling. Radiology. 1999;211:249–56.
Dimitrakopoulou-Strauss A, Strauss LG, Burger C, et al. Prognostic aspects of 18F-FDG PET kinetics in patients with metastatic colorectal carcinoma receiving FOLFOX chemotherapy. J Nucl Med. 2004;45:1480–7.
Dimitrakopoulou-Strauss A, Strauss LG, Egerer G, et al. Prediction of chemotherapy outcome in patients with metastatic soft tissue sarcomas based on dynamic FDG PET (dPET) and a multiparameter analysis. Eur J Nucl Med Mol Imaging. 2010;37:1481–9.
Dunnwald LK, Doot RK, Specht JM, et al. PET tumor metabolism in locally advanced breast cancer patients undergoing neoadjuvant chemotherapy: value of static versus kinetic measures of fluorodeoxyglucose uptake. Clin Cancer Res. 2011;17:2400–9.
Xiao Z, Dai Z, Locasale JW. Metabolic landscape of the tumor microenvironment at single cell resolution. Nat Commun. 2019;10:3763.
Reinfeld BI, Madden MZ, Wolf MM, et al. Cell-programmed nutrient partitioning in the tumour microenvironment. Nature. 2021;593:282–8.
Bodor JN, Boumber Y, Borghaei H. Biomarkers for immune checkpoint inhibition in non-small cell lung cancer (NSCLC). Cancer. 2020;126:260–70.
Mao W, Cai Y, Chen D, et al. Statin shapes inflamed tumor microenvironment and enhances immune checkpoint blockade in non-small cell lung cancer. JCI Insight. 2022;7(18):e161940.
Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13.
Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol. 2013;14:986–95.
Jabbour SK, Lee KH, Frost N, et al. Pembrolizumab Plus Concurrent Chemoradiation Therapy in Patients With Unresectable, Locally Advanced, Stage III Non-Small Cell Lung Cancer: The Phase 2 KEYNOTE-799 Nonrandomized Trial. JAMA Oncol. 2021;7:1–9.
Funding
This study was supported by the National Natural Science Foundation of China (Grant Number 82073328).
Author information
Authors and Affiliations
Contributions
HL, WF, DQW and BQ contributed to the study conception and design. All authors contributed to the patient recruitment, data collection and interpretation. HL, DQW, CJZ and SRL contributed to the data analysis, manuscript draft, review and editing. All the other authors were involved in patient recruitment and data curation. All authors contributed to the development of the article and approved the final version.
Corresponding authors
Ethics declarations
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the institutional Ethics Committee(20201126/A2020-011).
Consent to participate
Written informed consent was obtained from all individual participants included in the study.
Consent to publish
The authors affirm that human research participants provided informed consent for publication of the images.
Conflicts of interest
The authors have no potential conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, D., Qiu, B., Liu, Q. et al. Patlak-Ki derived from ultra-high sensitivity dynamic total body [18F]FDG PET/CT correlates with the response to induction immuno-chemotherapy in locally advanced non-small cell lung cancer patients. Eur J Nucl Med Mol Imaging 50, 3400–3413 (2023). https://doi.org/10.1007/s00259-023-06298-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-023-06298-x