Abstract
Purpose
To provide a theory for guiding clinical treatment by comparing the clinical application value of [18F]fluorodeoxyglucose ([18F]FDG) PET/CT and [68Ga]Ga-FAPI (fibroblast activating protein inhibitor) PET/MR in the diagnosis and evaluation of resectability of ovarian cancer.
Methods
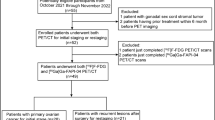
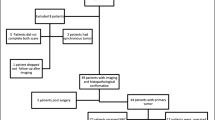
Thirty patients with high clinical suspicion of ovarian malignancies were enrolled from July 2021 to October 2022 and underwent [18F]FDG PET/CT and [68Ga]Ga-FAPI-04 PET/MR within 5 days. Twenty patients underwent [18F]FDG PET/MR at once completing [18F]FDG PET/CT for consistency checking. Images were analysed for comparing SUVs and for judging incomplete resectability according to the peritoneal cancer index (PCI) and SUIDAN scoring system. The expression of FAP, HK2 and Ki67 was analysed by immunohistochemistry staining.
Results
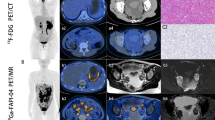
There was no significant difference between PET/MR and PET/CT in SUVs-FDG at different locations (p > 0.05), and their diagnostic accuracies were similar. The diagnostic accuracy of [68Ga]Ga-FAPI-04 PET/MR had advantages for peritoneal metastasis since SUVsFAPI were higher (p < 0.01). The sensitivity of [68Ga]Ga-FAPI-04 PET/MR in the diagnosis of peridiaghragmatic metastases was higher because SUVmax in the liver was decreased (p < 0.001). [68Ga]Ga-FAPI-04 PET/MR might have advantages in diagnosing gastrointestinal invasion. In PCI score analysis, [68Ga]Ga-FAPI-04 PET/MR could partially correct missing or underestimated scores by [18F]FDG PET/CT, but the matching probability between left peri-intestinal metastasis scores was low and easy to overestimate. Interestingly, diaphragmatic metastasis detected by [68Ga]Ga-FAPI-04 PET/MR had the greatest correlation with the prediction of incomplete resectability (logistic regression p = 0.02). Through immunohistochemistry, the expression of FAP had a strong correlation with SUVmax-FAPI (p < 0.001), while the expression of HK2 was correlated with SUVmax-FDG (p < 0.01). In addition, SUVmax-FDG with Ki67 ≥ 20% was significantly higher than that with Ki67 < 20% (p < 0.05).
Conclusions
[68Ga]Ga-FAPI-04 PET/MR had obvious advantages for metastases diagnosis and could more accurately assess tumour load and predict incomplete resectability. SUVmax-FDG was conducive to evaluating the degree of tumour malignancy.





Similar content being viewed by others
Data Availability
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
Abbreviations
- PCI:
-
Peritoneal cancer index
- EOC:
-
Epithelial ovarian cancer
- PDS:
-
Primary debulking surgery
- IDS:
-
Interval debulking surgery
- NACT:
-
Chemotherapy and neoadjuvant chemotherapy
- NCCN:
-
National Comprehensive Cancer Network
- US:
-
Ultrasonography
- PET:
-
Positron emission computed tomography
- [18F]FDG:
-
[18F]fluorodeoxyglucose
- [18F]FDG-6-PO4 :
-
[18F]fluorodeoxyglucose-6-phosphate
- HK:
-
Hexokinase
- FAP:
-
Fibroblast activating protein
- FAPI:
-
68Ga-Fibroblast activating protein inhibitor
- CAFs:
-
Cancer-related fibroblasts
- NAFs:
-
Normal activated fibroblasts
- ROI:
-
Region of interest
- TBR:
-
Tumour background ratio
- AUC:
-
Area under the curve
References
Armstrong DK, Alvarez RD, Bakkum-Gamez JN, Barroilhet L, Behbakht K, Berchuck A, et al. Ovarian cancer, version 2.2020, NCCN clinical practice guidelines in oncology. Journal of the National Comprehensive Cancer Network: JNCCN. 2021;19:191–226. https://doi.org/10.6004/jnccn.2021.0007.
Chi DS, Eisenhauer EL, Zivanovic O, Sonoda Y, Abu-Rustum NR, Levine DA, et al. Improved progression-free and overall survival in advanced ovarian cancer as a result of a change in surgical paradigm. Gynecol Oncol. 2009;114:26–31. https://doi.org/10.1016/j.ygyno.2009.03.018.
Kehoe S, Hook J, Nankivell M, Jayson GC, Kitchener H, Lopes T, et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet. 2015;386:249–57. https://doi.org/10.1016/S0140-6736(14)62223-6.
Harter P, Sehouli J, Vergote I, Ferron G, Reuss A, Meier W, et al. Randomized trial of cytoreductive surgery for relapsed ovarian cancer. N Engl J Med. 2021;385:2123–31. https://doi.org/10.1056/NEJMoa2103294.
Outwater EK, Dunton CJ. Imaging of the ovary and adnexa: clinical issues and applications of MR imaging. Radiology. 1995;194:1–18. https://doi.org/10.1148/radiology.194.1.7997533.
Grab D, Flock F, Stohr I, Nussle K, Rieber A, Fenchel S, et al. Classification of asymptomatic adnexal masses by ultrasound, magnetic resonance imaging, and positron emission tomography. Gynecol Oncol. 2000;77:454–9. https://doi.org/10.1006/gyno.2000.5768.
Warburg O. On the origin of cancer cells. Science. 1956;123:309–14. https://doi.org/10.1126/science.123.3191.309.
Khiewvan B, Torigian DA, Emamzadehfard S, Paydary K, Salavati A, Houshmand S, et al. An update on the role of PET/CT and PET/MRI in ovarian cancer. Eur J Nucl Med Mol Imaging. 2017;44:1079–91. https://doi.org/10.1007/s00259-017-3638-z.
Giesel FL, Kratochwil C, Lindner T, Marschalek MM, Loktev A, Lehnert W, et al. (68)Ga-FAPI PET/CT: biodistribution and preliminary dosimetry estimate of 2 DOTA-containing FAP-targeting agents in patients with various cancers. J Nucl Med. 2019;60:386–92. https://doi.org/10.2967/jnumed.118.215913.
Climent MT, Serra A, Gilabert-Estelles J, Gilabert-Aguilar J, Llueca A. Comparison of peritoneal carcinomatosis scoring methods in predicting resectability and prognosis in gynecologic malignancies. J Clin Med. 2021;10:2553. https://doi.org/10.3390/jcm10122553.
Wang J, Liu L, Pang H, Liu L, Jing X, Li Y. Preoperative PET/CT score can predict incomplete resection after debulking surgery for advanced serous ovarian cancer better than CT score, MTV, tumor markers and hematological markers. Acta Obstet Gynecol Scand. 2022;101:1315–27. https://doi.org/10.1111/aogs.14442.
Xi Y, Guo R, Hu J, Zhang M, Zhang X, Li B. 18F-fluoro-2-deoxy-D-glucose retention index as a prognostic parameter in patients with pancreatic cancer. Nucl Med Commun. 2014;35:1112–8. https://doi.org/10.1097/MNM.0000000000000178.
Rohrich M, Naumann P, Giesel FL, Choyke PL, Staudinger F, Wefers A, et al. Impact of (68)Ga-FAPI PET/CT imaging on the therapeutic management of primary and recurrent pancreatic ductal adenocarcinomas. J Nucl Med. 2021;62:779–86. https://doi.org/10.2967/jnumed.120.253062.
Serfling S, Zhi Y, Schirbel A, Lindner T, Meyer T, Gerhard-Hartmann E, et al. Improved cancer detection in Waldeyer’s tonsillar ring by (68)Ga-FAPI PET/CT imaging. Eur J Nucl Med Mol Imaging. 2021;48:1178–87. https://doi.org/10.1007/s00259-020-05055-8.
Miao Y, Zhang LF, Zhang M, Guo R, Liu MF, Li B. Therapeutic delivery of miR-143 targeting tumor metabolism in poorly differentiated thyroid cancer xenografts and efficacy evaluation using (18)F-FDG microPET-CT. Hum Gene Ther. 2019;30:882–92. https://doi.org/10.1089/hum.2018.160.
Incoronato M, Grimaldi AM, Cavaliere C, Inglese M, Mirabelli P, Monti S, et al. Relationship between functional imaging and immunohistochemical markers and prediction of breast cancer subtype: a PET/MRI study. Eur J Nucl Med Mol Imaging. 2018;45:1680–93. https://doi.org/10.1007/s00259-018-4010-7.
Thuillier P, Liberini V, Rampado O, Gallio E, De Santi B, Ceci F, et al. Diagnostic value of conventional PET parameters and radiomic features extracted from 18F-FDG-PET/CT for histologic subtype classification and characterization of lung neuroendocrine neoplasms. Biomedicines. 2021;9:281. https://doi.org/10.3390/biomedicines9030281.
Nelson BE, Rosenfield AT, Schwartz PE. Preoperative abdominopelvic computed tomographic prediction of optimal cytoreduction in epithelial ovarian carcinoma. J Clin Oncol. 1993;11:166–72. https://doi.org/10.1200/JCO.1993.11.1.166.
Axtell AE, Lee MH, Bristow RE, Dowdy SC, Cliby WA, Raman S, et al. Multi-institutional reciprocal validation study of computed tomography predictors of suboptimal primary cytoreduction in patients with advanced ovarian cancer. J Clin Oncol: Official J Am Soc Clin Oncol. 2007;25:384–9. https://doi.org/10.1200/JCO.2006.07.7800.
Suidan RS, Ramirez PT, Sarasohn DM, Teitcher JB, Mironov S, Iyer RB, et al. A multicenter prospective trial evaluating the ability of preoperative computed tomography scan and serum CA-125 to predict suboptimal cytoreduction at primary debulking surgery for advanced ovarian, fallopian tube, and peritoneal cancer. Gynecol Oncol. 2014;134:455–61. https://doi.org/10.1016/j.ygyno.2014.07.002.
Son HM, Kim SH, Kwon BR, Kim MJ, Kim CS, Cho SH. Preoperative prediction of suboptimal resection in advanced ovarian cancer based on clinical and CT parameters. Acta Radiol. 2017;58:498–504. https://doi.org/10.1177/0284185116658683.
Xi Y, Yuan P, Li T, Zhang M, Liu MF, Li B. hENT1 reverses chemoresistance by regulating glycolysis in pancreatic cancer. Cancer Lett. 2020;479:112–22. https://doi.org/10.1016/j.canlet.2020.03.015.
Shi T, Zhu J, Feng Y, Tu D, Zhang Y, Zhang P, et al. Secondary cytoreduction followed by chemotherapy versus chemotherapy alone in platinum-sensitive relapsed ovarian cancer (SOC-1): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2021;22:439–49. https://doi.org/10.1016/S1470-2045(21)00006-1.
Chen Y, McAndrews KM, Kalluri R. Clinical and therapeutic relevance of cancer-associated fibroblasts. Nat Rev Clin Oncol. 2021;18:792–804. https://doi.org/10.1038/s41571-021-00546-5.
Martinez-Outschoorn UE, Balliet R, Lin Z, Whitaker-Menezes D, Birbe RC, Bombonati A, et al. BRCA1 mutations drive oxidative stress and glycolysis in the tumor microenvironment: implications for breast cancer prevention with antioxidant therapies. Cell Cycle. 2012;11:4402–13. https://doi.org/10.4161/cc.22776.
Hwang RF, Moore T, Arumugam T, Ramachandran V, Amos KD, Rivera A, et al. Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Can Res. 2008;68:918–26. https://doi.org/10.1158/0008-5472.CAN-07-5714.
Kratochwil C, Flechsig P, Lindner T, Abderrahim L, Altmann A, Mier W, et al. (68)Ga-FAPI PET/CT: tracer uptake in 28 different kinds of cancer. J Nucl Med. 2019;60:801–5. https://doi.org/10.2967/jnumed.119.227967.
Chen H, Pang Y, Wu J, Zhao L, Hao B, Wu J, et al. Comparison of [(68)Ga]Ga-DOTA-FAPI-04 and [(18)F] FDG PET/CT for the diagnosis of primary and metastatic lesions in patients with various types of cancer. Eur J Nucl Med Mol Imaging. 2020;47:1820–32. https://doi.org/10.1007/s00259-020-04769-z.
Shangguan C, Gan G, Zhang J, Wu J, Miao Y, Zhang M, et al. Cancer-associated fibroblasts enhance tumor (18)F-FDG uptake and contribute to the intratumor heterogeneity of PET-CT. Theranostics. 2018;8:1376–88. https://doi.org/10.7150/thno.22717.
Ding J, Qiu J, Hao Z, Huang H, Liu Q, Liu W, et al. Prognostic value of preoperative [(68) Ga]Ga-FAPI-04 PET/CT in patients with resectable pancreatic ductal adenocarcinoma in correlation with immunohistological characteristics. Eur J Nucl Med Mol Imaging. 2023. https://doi.org/10.1007/s00259-022-06100-4.
Buck A, Schirrmeister H, Kuhn T, Shen C, Kalker T, Kotzerke J, et al. FDG uptake in breast cancer: correlation with biological and clinical prognostic parameters. Eur J Nucl Med Mol Imaging. 2002;29:1317–23. https://doi.org/10.1007/s00259-002-0880-8.
Tchou J, Sonnad SS, Bergey MR, Basu S, Tomaszewski J, Alavi A, et al. Degree of tumor FDG uptake correlates with proliferation index in triple negative breast cancer. Mol Imag Biol. 2010;12:657–62. https://doi.org/10.1007/s11307-009-0294-0.
Funding
This study was supported by the National Natural Science Foundation of China (grant number 81801728 from Author Yun Xi; grant number 82172601 from author Weiwei Feng), the Natural Science Foundation of Shanghai Science and Technology Commission (grant number 20ZR1433700 from author Weiwei Feng), and the Shanghai Municipal Key Clinical Specialty (grant number shslczdzk03403 from author Biao Li).
Author information
Authors and Affiliations
Contributions
Weiwei Feng and Biao Li designed the study. Yun Xi and Lili Sun performed the experiments and wrote the manuscript. Xiaoxia Che, Hua Liu, and Qun Wang selected cases and performed the clinical evaluation. Qian Qu and Wangxi Hai prepared radionuclides. Hongping Meng and Yuxin Miao operated PET/MR and PET/CT. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Ethics approval
This clinical investigation was approved by the Medical Ethical Committee of Shanghai Jiao Tong University Medical School Affiliated Ruijin Hospital (N2021-284).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Oncology - Genitourinary.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xi, Y., Sun, L., Che, X. et al. A comparative study of [68Ga]Ga-FAPI-04 PET/MR and [18F]FDG PET/CT in the diagnostic accuracy and resectability prediction of ovarian cancer. Eur J Nucl Med Mol Imaging 50, 2885–2898 (2023). https://doi.org/10.1007/s00259-023-06235-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-023-06235-y