Abstract
Purpose
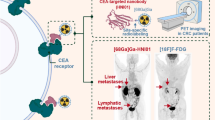
Here, we aim to identify a CEACAM5-targeted nanobody and demonstrate its application in positron emission tomography (PET) imaging and near-infrared (NIR) fluorescence imaging in colorectal cancer (CRC).
Methods
Immunohistochemistry was applied to verify CEACAM5 expression in CRC and metastatic lymph nodes (mLNs). CEACAM5-targeted nanobodies were obtained by immunization of human CEACAM5 protein in a dromedary, followed by several rounds of phage screenings. Immunofluorescence staining and flow cytometry was carried out to determine the binding affinity of the nanobodies. The nanobodies were radiolabeled by coupling 18F-SFB for PET imaging of CRC subcutaneous xenografts and lymph node metastasis (LNM). IRDye800CW (IR800) were conjugated to form NIR probes for NIR imaging in CRC subcutaneous models.
Results
CEACAM5 was overexpressed in either human CRC tissues or mLNs. A CEACAM5 targeted nanobody, Nb41 was successfully generated, with excellent in vitro binding properties. Incorporation of albumin binding domain (ABD) did not affect the affinity of Nb41. In vivo imaging showed that both 18F-FB-Nb41 and 18F-FB-Nb41-ABD showed obvious accumulation in the tumor. Due to the longer retention in the blood, 18F-FB-Nb41-ABD enrichment in tumors was significantly delayed but higher compared to 18F-FB-Nb41. Both 18F-FB-Nb41 and 18F-FB-Nb41-ABD showed prominent LNM enrichment. Similarly, the IR800-conjugated nanobodies Nb41-IR800 and Nb41-ABD-IR800 exhibited superior imaging effects in subcutaneous models, while Nb41-ABD-IR800 exhibited higher fluorescence intensity in the tumor accompanied with a remarkedly delay compared to Nb41-IR800.
Conclusion
Collectively, we presented the identification and in vivo validation of a CEACAM5-targeted nanobody and a fused nanobody with an ABD, which enabled to the non-invasive visualization of malignancy of CRC using PET imaging and NIR imaging in subcutaneous models as well as LNM models.






Similar content being viewed by others
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. https://doi.org/10.3322/caac.21492.
Ulintz PJ, Greenson JK, Wu R, Fearon ER, Hardiman KM. Lymph Node Metastases in Colon Cancer Are Polyclonal. Clin Cancer Res. 2018;24(9):2214–24. https://doi.org/10.1158/1078-0432.CCR-17-1425.
Rigo P, Paulus P, Kaschten BJ, Hustinx R, Bury T, Jerusalem G, et al. Oncological applications of positron emission tomography with fluorine-18 fluorodeoxyglucose. Eur J Nucl Med. 1996;23(12):1641–74. https://doi.org/10.1007/BF01249629.
Kostakoglu L, Hardoff R, Mirtcheva R, Goldsmith SJ. PET-CT fusion imaging in differentiating physiologic from pathologic FDG uptake. Radiographics. 2004;24(5):1411–31. https://doi.org/10.1148/rg.245035725.
Te VE, Veerman T, Subramaniam V, Ruers T. The use of fluorescent dyes and probes in surgical oncology. Eur J Surg Oncol. 2010;36(1):6–15. https://doi.org/10.1016/j.ejso.2009.10.014.
Barnett T, Goebel SJ, Nothdurft MA, Elting JJ. Carcinoembryonic antigen family: characterization of cDNAs coding for NCA and CEA and suggestion of nonrandom sequence variation in their conserved loop-domains. Genomics. 1988;3(1):59–66. https://doi.org/10.1016/0888-7543(88)90160-7.
Gold P, Freedman SO. Specific carcinoembryonic antigens of the human digestive system. J Exp Med. 1965;122(3):467–81. https://doi.org/10.1084/jem.122.3.467.
Colcher D, Esteban J, Mornex F. Use of monoclonal antibodies as radiopharmaceuticals for the localization of human carcinoma xenografts in athymic mice. Methods Enzymol. 1986;121:802–16. https://doi.org/10.1016/0076-6879(86)21078-2.
Liang M, Yang M, Wang F, Wang X, He B, Mei C, et al. Near-infrared fluorescence-guided resection of micrometastases derived from esophageal squamous cell carcinoma using a c-Met-targeted probe in a preclinical xenograft model. J Control Release. 2021;332:171–83. https://doi.org/10.1016/j.jconrel.2021.02.019.
Wang L, Liang M, Xiao Y, Chen J, Mei C, Lin Y, et al. NIR-II Navigation with an EGFR-Targeted Probe Improves Imaging Resolution and Sensitivity of Detecting Micrometastases in Esophageal Squamous Cell Carcinoma Xenograft Models. Mol Pharm. 2022;19(10):3563–75. https://doi.org/10.1021/acs.molpharmaceut.2c00115.
Xiao YT, Zhou C, Ye JC, Yang XC, Li ZJ, Zheng XB, et al. Integrin alpha6-Targeted Positron Emission Tomography Imaging of Colorectal Cancer. ACS Omega. 2019;4(13):15560–6. https://doi.org/10.1021/acsomega.9b01920.
Mcmahon C, Baier AS, Pascolutti R, Wegrecki M, Zheng S, Ong JX, et al. Yeast surface display platform for rapid discovery of conformationally selective nanobodies. Nat Struct Mol Biol. 2018;25(3):289–96. https://doi.org/10.1038/s41594-018-0028-6.
Wolfson W. Ablynx makes nanobodies from llama bodies. Chem Biol. 2006;13(12):1243–4. https://doi.org/10.1016/j.chembiol.2006.12.003.
Dennis MS, Jin H, Dugger D, Yang R, Mcfarland L, Ogasawara A, et al. Imaging tumors with an albumin-binding Fab, a novel tumor-targeting agent. Cancer Res. 2007;67(1):254–61. https://doi.org/10.1158/0008-5472.CAN-06-2531.
Vincke C, Gutiérrez C, Wernery U, Devoogdt N, Hassanzadeh-Ghassabeh G, Muyldermans S. Generation of Single Domain Antibody Fragments Derived from Camelids and Generation of Manifold Constructs. In: Chames P, editor. Antibody Engineering: Methods and Protocols. 2nd ed. Totowa, NJ: Humana Press; 2012. p. 145–76.
Hatanaka K, Asai T, Koide H, Kenjo E, Tsuzuku T, Harada N, et al. Development of double-stranded siRNA labeling method using positron emitter and its in vivo trafficking analyzed by positron emission tomography. Bioconjug Chem. 2010;21(4):756–63. https://doi.org/10.1021/bc9005267.
Vaidyanathan G, Zalutsky MR. Synthesis of N-succinimidyl 4-[18F]fluorobenzoate, an agent for labeling proteins and peptides with 18F. Nat Protoc. 2006;1(4):1655–61. https://doi.org/10.1038/nprot.2006.264.
Wei J, Bera TK, Liu XF, Zhou Q, Onda M, Ho M, et al. Recombinant immunotoxins with albumin-binding domains have long half-lives and high antitumor activity. Proc Natl Acad Sci U S A. 2018;115(15):E3501–8. https://doi.org/10.1073/pnas.1721780115.
de Gooyer JM, Elekonawo F, Bos DL, van der Post RS, Pelegrin A, Framery B, et al. Multimodal CEA-Targeted Image-Guided Colorectal Cancer Surgery using (111)In-Labeled SGM-101. Clin Cancer Res. 2020;26(22):5934–42. https://doi.org/10.1158/1078-0432.CCR-20-2255.
Wang Y, Lin Q, Shi H, Cheng D. Fluorine-18: Radiochemistry and Target-Specific PET Molecular Probes Design. Front Chem. 2022;10:884517. https://doi.org/10.3389/fchem.2022.884517.
Qin X, Guo X, Liu T, Li L, Zhou N, Ma X, et al. High in-vivo stability in preclinical and first-in-human experiments with [(18)F]AlF-RESCA-MIRC213: a (18)F-labeled nanobody as PET radiotracer for diagnosis of HER2-positive cancers. Eur J Nucl Med Mol Imaging. 2023;50(2):302–13. https://doi.org/10.1007/s00259-022-05967-7.
Wang C, Chen Y, Hou YN, Liu Q, Zhang D, Zhao H, et al. ImmunoPET imaging of multiple myeloma with [(68)Ga]Ga-NOTA-Nb1053. Eur J Nucl Med Mol Imaging. 2021;48(9):2749–60. https://doi.org/10.1007/s00259-021-05218-1.
Warnders FJ, Terwisscha VSA, Knuehl C, van Roy M, de Vries E, Kosterink J, et al. Human Epidermal Growth Factor Receptor 3-Specific Tumor Uptake and Biodistribution of (89)Zr-MSB0010853 Visualized by Real-Time and Noninvasive PET Imaging. J Nucl Med. 2017;58(8):1210–5. https://doi.org/10.2967/jnumed.116.181586.
Liu Q, Jiang L, Li K, Li H, Lv G, Lin J, et al. Immuno-PET imaging of (68)Ga-labeled nanobody Nb109 for dynamic monitoring the PD-L1 expression in cancers. Cancer Immunol Immunother. 2021;70(6):1721–33. https://doi.org/10.1007/s00262-020-02818-y.
D’Huyvetter M, Vincke C, Xavier C, Aerts A, Impens N, Baatout S, et al. Targeted radionuclide therapy with A 177Lu-labeled anti-HER2 nanobody. Theranostics. 2014;4(7):708–20. https://doi.org/10.7150/thno.8156.
Wei W, Rosenkrans ZT, Liu J, Huang G, Luo QY, Cai W. ImmunoPET: Concept, Design, and Applications. Chem Rev. 2020;120(8):3787–851. https://doi.org/10.1021/acs.chemrev.9b00738.
Wada Y, Shimada M, Murano T, Takamaru H, Morine Y, Ikemoto T, et al. A Liquid Biopsy Assay for Noninvasive Identification of Lymph Node Metastases in T1 Colorectal Cancer. Gastroenterology. 2021;161(1):151-162.e1. https://doi.org/10.1053/j.gastro.2021.03.062.
Jin M, Frankel WL. Lymph Node Metastasis in Colorectal Cancer. Surg Oncol Clin N Am. 2018;27(2):401–12. https://doi.org/10.1016/j.soc.2017.11.011.
Tamaru Y, Oka S, Tanaka S, Nagata S, Hiraga Y, Kuwai T, et al. Long-term outcomes after treatment for T1 colorectal carcinoma: a multicenter retrospective cohort study of Hiroshima GI Endoscopy Research Group. J Gastroenterol. 2017;52(11):1169–79. https://doi.org/10.1007/s00535-017-1318-1.
Heriot AG, Hicks RJ, Drummond EG, Keck J, Mackay J, Chen F, et al. Does positron emission tomography change management in primary rectal cancer? A prospective assessment Dis Colon Rectum. 2004;47(4):451–8. https://doi.org/10.1007/s10350-003-0089-3.
Kijanka M, Warnders FJ, El KM, Lub-De HM, van Dam GM, Ntziachristos V, et al. Rapid optical imaging of human breast tumour xenografts using anti-HER2 VHHs site-directly conjugated to IRDye 800CW for image-guided surgery. Eur J Nucl Med Mol Imaging. 2013;40(11):1718–29. https://doi.org/10.1007/s00259-013-2471-2.
Hammarstrom S. The carcinoembryonic antigen (CEA) family: structures, suggested functions and expression in normal and malignant tissues. Semin Cancer Biol. 1999;9(2):67–81. https://doi.org/10.1006/scbi.1998.0119.
Shah NN, Fry TJ. Mechanisms of resistance to CAR T cell therapy. Nat Rev Clin Oncol. 2019;16(6):372–85. https://doi.org/10.1038/s41571-019-0184-6.
Acknowledgements
We appreciate Shaohui Deng and Yuanqiang Xiao for the assistance in the synthesis of precursor compounds. We are grateful to Tianxing Zhu and Jiayi Jiang for technical support of MRI. We would like to express our sincere gratitude to Guolong Huang for his help in PET imaging.
Funding
This work was supported by grants from the National Natural Science Foundation of China (92259204) and the Department of Science and Technology of Guangdong Province to the Guangdong Provincial Key Laboratory of Biomedical Imaging (2018B030322006).
Author information
Authors and Affiliations
Contributions
Conception: Dan Li, Pengfei Pang, Junjie Mao. Methodology: Yitai Xiao, Chaoming Mei, Duo Xu, Fan Yang, Meilin Yang. Data acquisition and analysis: Yitai Xiao, Chaoming Mei, Duo Xu, Lei Bi. Manuscript preparation and revision: Yitai Xiao, Chaoming Mei, Duo Xu. Study supervision: Dan Li.
Corresponding authors
Ethics declarations
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
The animal experiments were reviewed and approved by the animal welfare and ethics committees of the Fifth Affiliated Hospital of Sun Yat-sen University. Approval for the use of human samples was obtained from the Ethics Review Committee of the Fifth Affiliated Hospital of Sun Yat-sen University with waiver of informed consents (K170-1).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Preclinical Imaging.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xiao, Y., Mei, C., Xu, D. et al. Identification of a CEACAM5 targeted nanobody for positron emission tomography imaging and near-infrared fluorescence imaging of colorectal cancer. Eur J Nucl Med Mol Imaging 50, 2305–2318 (2023). https://doi.org/10.1007/s00259-023-06183-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-023-06183-7