Abstract
Introduction
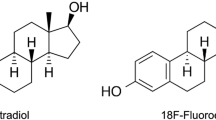
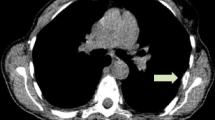
[18F]Fluoroestradiol ([18F]FES) PET/CT has been proposed as a tool for detecting the oestrogen receptor density in patients with metastatic breast cancer (BC) non-invasively across all disease localizations. However, its diagnostic potential in terms of the detection rate (DR) of metastases is unclear. In this study, we pitted this method against [18F]FDG PET/CT and tried to identify predictors of the diagnostic superiority of the [18F] FES-based method.
Materials and methods
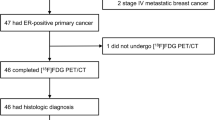
From a multicentre database, we enrolled all patients with metastatic BC who had undergone both [18F]FES PET/CT and [18F]FDG PET/CT. Two readers assessed both images independently and used a patient-based (PBA) and lesion-based analysis (LBA) to calculate the DR. Pathology-related and clinical factors were tested as predictors of [18F]FES PET/CT superiority using a multivariate model.
Results
92 patients, bearing a total of 2678 metastases, were enrolled. On PBA, the DR of [18F]FDG and [18F]FES PET/CT was 97% and 86%, respectively (p = 0.018). On LBA, the [18F]FES method proved more sensitive than [18F]FDG PET/CT in lymph nodes, bone, lung and soft tissue (p < 0.01). This greater sensitivity was associated with lobular histology, both on PBA (Odds Ratio (OR) 3.4, 95%CI 1.0–12.3) and on LBA (OR 4.4, 95%CI 1.2–16.1 for lymph node metastases and OR 3.29, 95%CI 1.1–10.2 for bone localizations).
Conclusions
The overall DR of [18F]FES PET/CT appears to be lower than that of [18F]FDG PET/CT on PBA. However, the [18F]FES method, if positive, can identify more lesions than [18F]FDG at most sites. The higher sensitivity of [18F]FES PET/CT was associated with lobular histology.

Similar content being viewed by others
Data availability
The datasets analysed during the current study are available from the corresponding author upon reasonable request.
References
Pabst KM, Decker T, Kersting D, Bartel T, Sraieb M, Herrmann K, et al. The future role of PET imaging in metastatic breast cancer. Oncol Res Treat. 2022; 45:18–25.
Chae SY, Ahn SH, Kim SB, Han S, Lee SH, Oh SJ, et al. Diagnostic accuracy and safety of 16α-[ 18 F]fluoro-17β-oestradiol PET-CT for the assessment of oestrogen receptor status in recurrent or metastatic lesions in patients with breast cancer: a prospective cohort study. Lancet Oncol. 2019;20:546–55.
van Geel JJL, Boers J, Elias SG, Glaudemans AWJM, de Vries EFJ, Hospers GAP, et al. Clinical validity of 16α-[ 18 F]Fluoro-17β-Estradiol positron emission tomography/computed tomography to assess estrogen receptor status in newly diagnosed metastatic breast cancer. J Clin Oncol. 2022;40:3642–52.
Evangelista L, Vittoria Dieci M, Guarneri V, Franco Conte P. 18F-Fluoroestradiol positron emission tomography in breast cancer patients: systematic review of the literature & meta-analysis. Curr Radiopharm. 2016;9:244–57
Gupta M, Datta A, Choudhury P, Dsouza M, Batra U, Mishra A. Can 18 F-Fluoroestradiol positron emission tomography become a new imaging standard in the estrogen receptor-positive breast cancer patient: a prospective comparative study with 18 F-Fluorodeoxyglucose positron emission tomography?. World J Nucl Med. 2017;16:133–9
Chae SY, Son HJ, Lee DY, Shin E, Oh JS, Seo SY, et al. Comparison of diagnostic sensitivity of [18F]fluoroestradiol and [18F]fluorodeoxyglucose positron emission tomography/computed tomography for breast cancer recurrence in patients with a history of estrogen receptor-positive primary breast cancer. EJNMMI Res. 2020;10:54.
Liu C, Gong C, Liu S, Zhang Y, Zhang Y, Xu X, et al. 18F-FES PET/CT influences the staging and management of patients with newly diagnosed estrogen receptor-positive breast cancer: a retrospective comparative study with 18F-FDG PET/CT. Oncologist. 2019;24: e1277–e1285.
Liu C, Xu X, Yuan H, Zhang Y, Zhang Y, Song S, et al. Dual Tracers of 16α-[18F]fluoro-17β-Estradiol and [18F]fluorodeoxyglucose for prediction of progression-free survival after fulvestrant therapy in patients with hr+/her2- metastatic breast cancer. Front Oncol. 2020;10:580277.
Bottoni G, Piccardo A, Fiz F, Siri G, Matteucci F, Rocca A, et al. Heterogeneity of bone metastases as an important prognostic factor in patients affected by oestrogen receptor-positive breast cancer. The role of combined [18F]Fluoroestradiol PET/CT and [18F]Fluorodeoxyglucose PET/CT. Eur J Radiol. 2021;141:109821.
Yang Z, Sun Y, Xue J, Yao Z, Xu J, Cheng J, et al. Can positron emission tomography/computed tomography with the dual tracers fluorine-18 fluoroestradiol and fluorodeoxyglucose predict neoadjuvant chemotherapy response of breast cancer? ----A Pilot Study. PLoS ONE. 2013;8.:e78192.
Ulaner GA, Jhaveri K, Chandarlapaty S, Hatzoglou V, Riedl CC, Lewis JS, et al. Head-to-Head evaluation of 18F-FES and 18F-FDG PET/CT in metastatic invasive lobular breast cancer. J Nucl Med. 2021;62:326–31.
Piccardo A, Fiz F, Treglia G, Bottoni G, Trimboli P. Head-to-Head comparison between18 F-FES PET/CT and18 F-FDG PET/CT in oestrogen receptor-positive breast cancer: a systematic review and meta-analysis. J Clin Med. 2022;11:1919.
Peterson LM, Kurland BF, Schubert EK, Link JM, Gadi VK, Specht JM, et al. A Phase 2 study of 16α-[18F]-fluoro-17β-estradiol positron emission tomography (FES-PET) as a marker of hormone sensitivity in metastatic breast cancer (MBC). Mol Imaging Biol. 2014;16:431–40.
Kurland BF, Peterson LM, Lee JH, Schubert EK, Currin ER, Link JM, et al. Estrogen receptor binding (18F-FES PET) and glycolytic activity (18F-FDG PET) predict progression-free survival on endocrine therapy in patients with ER+ breast cancer. Clin Cancer Res. 2017;23:407–15.
Boellaard R, Delgado-Bolton R, Oyen WJG, Giammarile F, Tatsch K, Eschner W, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42:328–54
van Kruchten M, Glaudemans AWJM, de Vries EFJ, Schröder CP, de Vries EGE, Hospers GAP. Positron emission tomography of tumour [18F]fluoroestradiol uptake in patients with acquired hormone-resistant metastatic breast cancer prior to oestradiol therapy. Eur J Nucl Med Mol Imaging. 2015;42:1674–81.
Boellaard R, Delgado-Bolton R, Oyen WJG, Giammarile F, Tatsch K, Eschner W, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42:328–54.
Mammatas LH, Venema CM, Schröder CP, de Vet HCW, van Kruchten M, Glaudemans AWJM, et al. Visual and quantitative evaluation of [18F]FES and [18F]FDHT PET in patients with metastatic breast cancer: an interobserver variability study. EJNMMI Res. 2020;10:40.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Monti M, Degenhardt T, Brain E, Wuerstlein R, Argusti A, Puntoni M, et al. ERANET JTC 2011: submission and activation of an international academic translational project in advanced breast cancer. Experience From the ET-FES Study. Front Med (Lausanne). 2022;8:817678.
Lee SJ, Park S, Ahn HK, Yi JH, Cho EY, Sun JM, et al. Implications of bone-only metastases in breast cancer: Favorable preference with excellent outcomes of hormone receptor positive breast cancer. Cancer Res Treat. 2011;43:89–95.
Ji L, Cheng L, Zhu X, Gao Y, Fan L, Wang Z. Risk and prognostic factors of breast cancer with liver metastases. BMC Cancer. 2021;21:238.
Hogan MP, Goldman DA, Dashevsky B, Riedl CC, Gönen M, Osborne JR, et al. Comparison of 18F-FDG PET/CT for systemic staging of newly diagnosed invasive lobular carcinoma versus invasive ductal carcinoma. J Nucl Med. 2015;56:1674–80.
Mouabbi JA, Hassan A, Lim B, Hortobagyi GN, Tripathy D, Layman RM. Invasive lobular carcinoma: an understudied emergent subtype of breast cancer. Breast Cancer Res Treat. 2022;193:253–64.
Okcu O, Sen B, Ozturk C, Findik Guvendi G, Bedir R. GLUT-1 expression in breast cancer. Turk Patoloji Derg. 2022;38:114–21.
Aide N, Lasnon C, Veit-Haibach P, Sera T, Sattler B, Boellaard R. EANM/EARL harmonization strategies in PET quantification: from daily practice to multicentre oncological studies. Eur J Nucl Med Mol Imaging. 2017;44:17–31
Funding
This research received grants from “Associazione Italiana per la Ricerca sul Cancro” (AIRC 2013) and ERA-Net TRANSCAN JTC 2011 Italian Ministry of Health.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Gianluca Bottoni, Francesco Fiz and Arnoldo Piccardo. The first draft of the manuscript was written by Arnoldo Piccardo and Andrea DeCensi; all authors commented on previous versions of the manuscript.
All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
This study was approved by the local ethics committees and the public medical agencies of all the centres involved in this clinical trial.
Informed consent
Written informed consent was obtained from all patients.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Alessandra Gennari and Arnoldo Piccardo share senior co-authorship.
This article is part of the Topical Collection on Oncology - General
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bottoni, G., Fiz, F., Puntoni, M. et al. Diagnostic effectiveness of [18F]Fluoroestradiol PET/CT in oestrogen receptor-positive breast cancer: the key role of histopathology. Evidence from an international multicentre prospective study. Eur J Nucl Med Mol Imaging 50, 2477–2485 (2023). https://doi.org/10.1007/s00259-023-06173-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-023-06173-9