Abstract
Purpose
Pafolacianine, a folate receptor alpha-targeted NIR tracer, has demonstrated clear efficacy in intraoperative molecular imaging–guided (IMI) lung cancer surgery. However, the selection of patients who would benefit from IMI remains challenging given the variability of fluorescence with patient-associated and histopathologic factors. Our goal in this study was to prospectively evaluate whether preoperative FRα/FRβ staining can predict pafolacianine-based fluorescence during real-time lung cancer resections.
Methods
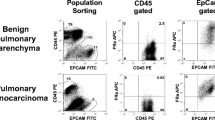
This was a prospective study conducted between 2018 and 2022 that reviewed core biopsy and intraoperative data from patients with suspected lung cancer. A total of 196 patients were deemed eligible, of whom core biopsies were taken from 38 patients and assessed for FRα and FRβ expression by immunohistochemistry (IHC). All patients underwent infusion of pafolacianine 24 h prior to surgery. Intraoperative fluorescence images were captured with the VisionSense bandpass filter–enabled camera. All histopathologic assessments were performed by a board-certified thoracic pathologist.
Results
Of the 38 patients, 5 (13.1%) were found to have benign lesions (necrotizing granulomatous inflammation, lymphoid aggregates) and 1 had metastatic non-lung nodule. Thirty (81.5%) had malignant lesions, with the vast majority (23, 77.4%) being lung adenocarcinoma (7 (22.5%) SCC). None of the benign tumors (0/5, 0%) exhibited in vivo fluorescence (mean TBR of 1.72), while 95% of the malignant tumors fluoresced (mean TBR of 3.11 ± 0.31) compared to squamous cell carcinoma (1.89 ± 0.29) of the lung and sarcomatous lung metastasis (2.32 ± 0.09) (p < 0.01). The TBR was significantly higher in the malignant tumors (p = 0.009). The median FRα and FRβ staining intensities were both 1.5 for benign tumors, while the FRα and FRβ staining intensities were 3 and 2 for malignant tumors, respectively. Increased FRα expression was significantly associated with the presence of fluorescence (p = 0.01),
Conclusion
This prospective study sought to determine whether preoperative FRα and FRβ expression on core biopsy IHC correlates with intraoperative fluorescence during pafolacianine-guided surgery. These results, although of small sample size, including limited non-adenocarcinoma cohort, suggest that performing FRα IHC on preoperative core biopsies of adenocarcinomas as compared to squamous cell carcinomas could provide low-cost, clinically useful information for optimal patient selection which should be further explored in advanced clinical trials.





Similar content being viewed by others
Data availability
All data is available in the manuscript and the supplementary file. Additional data can be obtained by contacting the corresponding author.
References
Thomas A, Chen Y, Yu T, Jakopovic M, Giaccone G. Trends and characteristics of young non-small cell lung cancer patients in the United States. Front Oncol. 2015;5:113.
Torre LA, Siegel RL, Jemal A. Lung cancer statistics. Adv Exp Med Biol. 2016;893:1–19.
Fedor D, Johnson WR, Singhal S. Local recurrence following lung cancer surgery: incidence, risk factors, and outcomes. Surg Oncol. 2013;22:156–61.
Azari F, Kennedy G, Singhal S. Intraoperative detection and assessment of lung nodules. Surgical Oncology Clinics Elsevier. 2020;29:525–41.
Borczuk AC. Challenges of frozen section in thoracic pathology: lepidic lesions, limited resections, and margins. Arch Pathol Lab Med. 2017;141:932–9.
Kennedy GT, Azari FS, Bernstein E, Desphande C, Din A, Marfatia I, et al. 3D specimen mapping expedites frozen section diagnosis of non-palpable ground glass opacities. The Annals of Thoracic Surgery: Elsevier; 2021.
Azari F, Zhang K, Kennedy GT, Chang A, Nadeem B, Delikatny EJ, et al. State of the art: precision surgery guided by intraoperative molecular imaging. Journal of Nuclear Medicine [Internet]. Society of Nuclear Medicine; 2022 [cited 2022 Aug 20]; Available from: https://jnm.snmjournals.org/content/early/2022/08/11/jnumed.121.263409. Accessed 1 Sep 2022
Azari F, Kennedy G, Bernstein E, Hadjipanayis CG, Vahrmeijer AL, Smith BL, et al. Intraoperative molecular imaging clinical trials: a review of 2020 conference proceedings. JBO. Int Soc Optics Photon. 2021;26:050901.
O’Shannessy DJ, Yu G, Smale R, Fu Y-S, Singhal S, Thiel RP, et al. Folate receptor alpha expression in lung cancer: diagnostic and prognostic significance. Oncotarget. 2012;3:414–25.
Predina JD, Newton AD, Connolly C, Dunbar A, Baldassari M, Deshpande C, et al. Identification of a folate receptor-targeted near-infrared molecular contrast agent to localize pulmonary adenocarcinomas. Mol Ther. 2018;26:390–403.
Kennedy GT, Okusanya OT, Keating JJ, Heitjan DF, Deshpande C, Litzky LA, et al. The optical biopsy: a novel technique for rapid intraoperative diagnosis of primary pulmonary adenocarcinomas. Ann Surg. 2015;262:602–9.
Randall LM, Wenham RM, Low PS, Dowdy SC, Tanyi JL. A phase II, multicenter, open-label trial of OTL38 injection for the intra-operative imaging of folate receptor-alpha positive ovarian cancer. Gynecol Oncol. 2019;155:63–8.
Predina JD, Newton AD, Keating J, Dunbar A, Connolly C, Baldassari M, et al. A phase I clinical trial of targeted intraoperative molecular imaging for pulmonary adenocarcinomas. Ann Thorac Surg. 2018;105:901–8.
Tie Y, Zheng H, He Z, Yang J, Shao B, Liu L, et al. Targeting folate receptor β positive tumor-associated macrophages in lung cancer with a folate-modified liposomal complex. Signal Transduction and Targeted Therapy. Nature Publishing Group; 2020;5:1–15.
Azari F, Kennedy G, Zhang K, Bernstein E, Chang A, Nadeem B, Segil A, Desphande C, Delikatny J, Kucharczuk J, Singhal S. Effects of Light-absorbing Carbons in Intraoperative Molecular Imaging-Guided Lung Cancer Resections. Mol Imaging Biol. 2023;25(1):156–167. https://doi.org/10.1007/s11307-021-01699-6
De Jesus E, Keating JJ, Kularatne SA, Jiang J, Judy R, Predina J, et al. Comparison of folate receptor targeted optical contrast agents for intraoperative molecular imaging. Int J Mol Imaging [Internet]. 2015 [cited 2020 Mar 7];2015. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4600912/. Accessed 1 Sept 2022
Paulos C. Folate receptor-mediated targeting of therapeutic and imaging agents to activated macrophages in rheumatoid arthritis. Adv Drug Deliv Rev. 2004;56:1205–17.
Azari F, Kennedy G, Bernstein E, Delikatny J, Lee JYK, Kucharczuk J, Low PS, Singhal S. Evaluation of OTL38-Generated Tumor-to-Background Ratio in Intraoperative Molecular Imaging-Guided Lung Cancer Resections. Mol Imaging Biol. 2023;25(1):85–96. https://doi.org/10.1007/s11307-021-01618-9.
Azari F, Kennedy G, Chang A, Nadeem B, Sullivan N, Marfatia I, Din A, Desphande C, Kucharczuk J, Delikatny EJ, Singhal S. Presence of non-Newtonian fluid in invasive pulmonary mucinous adenocarcinomas impacts fluorescence during intraoperative molecular imaging of lung cancer. Eur J Nucl Med Mol Imaging. 2022;49(13):4406–4418. https://doi.org/10.1007/s00259-022-05912-8.
Devarajan SR, Zarrin-Khameh N, Alapat P. Black lungs and big nodes: a case of airway anthracosis with bronchial anthracofibrosis. Respir Med Case Rep. 2018;25:9–11.
Chi C, Du Y, Ye J, Kou D, Qiu J, Wang J, et al. Intraoperative imaging-guided cancer surgery: from current fluorescence molecular imaging methods to future multi-modality imaging technology. Theranostics. 2014;4:1072–84.
Azari F, Kennedy GT, Chang A, Nadeem B, Bou-Samra P, Chang A, et al. Sodium multivitamin transporter-targeted fluorochrome facilitates enhanced metabolic evaluation of tumors through coenzyme-R dependent intracellular signaling pathways. Mol Imaging Biol [Internet]. 2022. https://doi.org/10.1007/s11307-022-01792-4.
Cui F, Liu J, Du M, Fan J, Fu J, Geng Q, et al. Expert consensus on indocyanine green fluorescence imaging for thoracoscopic lung resection (The Version 2022). Transl Lung Cancer Res. 2022;11:2318–31.
Bach PB, Mirkin JN, Oliver TK, Azzoli CG, Berry D, Brawley OW, et al. Benefits and harms of CT screening for lung cancer: a systematic review. JAMA. 2012;307:2418–29.
Cho E, Kang MH, Choi KS, Suh M, Jun JK, Park E-C. Cost-effectiveness outcomes of the national gastric cancer screening program in South Korea. Asian Pac J Cancer Prev. 2013;14:2533–40.
Kennedy GT, Azari FS, Bernstein E, Marfatia I, Din A, Kucharczuk JC, et al. Targeted intraoperative molecular imaging for localizing nonpalpable tumors and quantifying resection margin distances. JAMA Surgery [Internet]. 2021. https://doi.org/10.1001/jamasurg.2021.3757.
Udall M, Rizzo M, Kenny J, Doherty J, Dahm S, Robbins P, et al. PD-L1 diagnostic tests: a systematic literature review of scoring algorithms and test-validation metrics. Diagn Pathol. 2018;13:12.
Kennedy GT, Azari FS, Chang A, Nadeem B, Bernstein E, Segil A, et al. Comparative experience of short-wavelength versus long-wavelength fluorophores for intraoperative molecular imaging of lung cancer. Ann Surg. 2022;276:711.
Acknowledgements
Figure panels edited with BioRender.
Funding
Dr. Azari was supported by the training grant in Surgical Oncology by the National Institutes of Health (T32CA251063-01), the Society of Thoracic Surgeons Thoracic Surgery Foundation Research Award, and the Stephen CC Cheung Fellowship in Surgical Oncology. Dr. Singhal was supported by the National Institutes of Health (NIH P01CA254859) and the State of Pennsylvania Health Research Formula Fund. Dr. Kennedy was supported by the American Philosophical Society and the National Institutes of Health (grant F32 CA254210-01).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and approved by the University of Pennsylvania Institutional Review Board. The study complies with the national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Oncology—Chest.
Supplementary Information
Below is the link to the electronic supplementary material.
259_2023_6141_MOESM1_ESM.jpeg
Supplementary file1 Fig. 1: False-positive fluorescence of benign lymph nodes with IHC demonstrating strong FRβ density and minimal FRα presence. (JPEG 972 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Azari, F., Zhang, K., Kennedy, G. et al. Prospective validation of tumor folate receptor expression density with the association of pafolacianine fluorescence during intraoperative molecular imaging–guided lung cancer resections. Eur J Nucl Med Mol Imaging 50, 2453–2465 (2023). https://doi.org/10.1007/s00259-023-06141-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-023-06141-3