Abstract
Purpose
To evaluate the diagnostic accuracy of [18F]-DCFPyL PET/MRI radiomics for the prediction of pathological grade group in prostate cancer (PCa) in therapy-naïve patients.
Methods
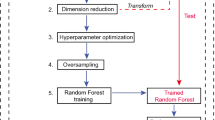
Patients with confirmed or suspected PCa, who underwent [18F]-DCFPyL PET/MRI (n = 105), were included in this retrospective analysis of two prospective clinical trials. Radiomic features were extracted from the segmented volumes following the image biomarker standardization initiative (IBSI) guidelines. Histopathology obtained from systematic and targeted biopsies of the PET/MRI-detected lesions was the reference standard. Histopathology patterns were dichotomized as ISUP GG 1–2 vs. ISUP GG ≥ 3 categories. Different single-modality models were defined for feature extraction, including PET- and MRI-derived radiomic features. The clinical model included age, PSA, and lesions’ PROMISE classification. Single models, as well as different combinations of them, were generated to calculate their performances. A cross-validation approach was used to evaluate the internal validity of the models.
Results
All radiomic models outperformed the clinical models. The best model for grade group prediction was the combination of PET + ADC + T2w radiomic features, showing sensitivity, specificity, accuracy, and AUC of 0.85, 0.83, 0.84, and 0.85, respectively. The MRI-derived (ADC + T2w) features showed sensitivity, specificity, accuracy, and AUC of 0.88, 0.78, 0.83, and 0.84, respectively. PET-derived features showed 0.83, 0.68, 0.76, and 0.79, respectively. The baseline clinical model showed 0.73, 0.44, 0.60, and 0.58, respectively. The addition of the clinical model to the best radiomic model did not improve the diagnostic performance. The performances of MRI and PET/MRI radiomic models as per the cross-validation scheme yielded an accuracy of 0.80 (AUC = 0.79), whereas clinical models presented an accuracy of 0.60 (AUC = 0.60).
Conclusion
The combined [18F]-DCFPyL PET/MRI radiomic model was the best-performing model and outperformed the clinical model for pathological grade group prediction, indicating a complementary value of the hybrid PET/MRI model for non-invasive risk stratification of PCa. Further prospective studies are required to confirm the reproducibility and clinical utility of this approach.



Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Siegel RL, et al. Cancer statistics, 2022. CA: A cancer journal for clinicians; 2022.
Litwin MS, Tan H-J. The diagnosis and treatment of prostate cancer: a review. JAMA. 2017;317(24):2532–42.
Epstein JI, et al. The 2014 International Society of Urological Pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma. Am J Surg Pathol. 2016;40(2):244–52.
Hamdy FC, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375:1415–24.
Wilt TJ, et al. Follow-up of prostatectomy versus observation for early prostate cancer. N Engl J Med. 2017;377(2):132–42.
Kasivisvanathan V, et al. MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med. 2018;378(19):1767–77.
Johnson DC, et al. Detection of individual prostate cancer foci via multiparametric magnetic resonance imaging. Eur Urol. 2019;75(5):712–20.
Schoots IG, et al. Magnetic resonance imaging–targeted biopsy may enhance the diagnostic accuracy of significant prostate cancer detection compared to standard transrectal ultrasound-guided biopsy: a systematic review and meta-analysis. Eur Urol. 2015;68(3):438–50.
Porpiglia F, et al. Diagnostic pathway with multiparametric magnetic resonance imaging versus standard pathway: results from a randomized prospective study in biopsy-naïve patients with suspected prostate cancer. Eur Urol. 2017;72(2):282–8.
Baco E, et al. A randomized controlled trial to assess and compare the outcomes of two-core prostate biopsy guided by fused magnetic resonance and transrectal ultrasound images and traditional 12-core systematic biopsy. Eur Urol. 2016;69(1):149–56.
Sonni I, et al. Head-to-head comparison of 68Ga-PSMA-11 PET/CT and mpMRI with a histopathology gold standard in the detection, intraprostatic localization, and determination of local extension of primary prostate cancer: results from a prospective single-center imaging trial. J Nucl Med. 2022;63(6):847–54.
Lambin P, et al. Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol. 2017;14(12):749–62.
Xu M, et al. Using biparametric MRI radiomics signature to differentiate between benign and malignant prostate lesions. Eur J Radiol. 2019;114:38–44.
Cysouw MC, et al. Machine learning-based analysis of [18F] DCFPyL PET radiomics for risk stratification in primary prostate cancer. Eur J Nucl Med Mol Imaging. 2021;48(2):340–9.
Yi Z, et al. Machine learning-based prediction of invisible intraprostatic prostate cancer lesions on 68 Ga-PSMA-11 PET/CT in patients with primary prostate cancer. Eur J Nucl Med Mol Imaging 2021;1–12.
Zamboglou C, et al. Radiomic features from PSMA PET for non-invasive intraprostatic tumor discrimination and characterization in patients with intermediate-and high-risk prostate cancer-a comparison study with histology reference. Theranostics. 2019;9(9):2595.
Solari EL, et al. The added value of PSMA PET/MR radiomics for prostate cancer staging. Eur J Nucl Med Mol Imaging. 2022;49(2):527–38.
Giesel FL, et al. Intraindividual comparison of 18F-PSMA-1007 and 18F-DCFPyL PET/CT in the prospective evaluation of patients with newly diagnosed prostate carcinoma: a pilot study. J Nucl Med. 2018;59(7):1076–80.
Morris MJ, et al. Diagnostic performance of 18F-DCFPyL-PET/CT in men with biochemically recurrent prostate cancer: results from the CONDOR Phase III. Multicenter StudyCONDOR trial Clinical Cancer Research. 2021;27(13):3674–82.
Metser U, et al. Effect of 18F-DCFPyL PET/CT on the management of patients with recurrent prostate cancer: results of a prospective multicenter registry trial. Radiology. 2022;303(2):414–22.
Basso Dias A, et al. Impact of 18F-DCFPyL PET on staging and treatment of unfavorable intermediate or high-risk prostate cancer. Radiology 2022;211836.
Guglielmo P, et al. Additional value of PET radiomic features for the initial staging of prostate cancer: a systematic review from the literature. Cancers. 2021;13(23):6026.
Ravert HT, et al. An improved synthesis of the radiolabeled prostate-specific membrane antigen inhibitor,[18F] DCFPyL. J Labelled Compd Radiopharm. 2016;59(11):439–50.
Metser U, et al. Comparison of MRI sequences in whole-body PET/MRI for staging of patients with high-risk prostate cancer. Am J Roentgenol. 2019;212(2):377–81.
Nioche C, et al. LIFEx: a freeware for radiomic feature calculation in multimodality imaging to accelerate advances in the characterization of tumor heterogeneity. Can Res. 2018;78(16):4786–9.
Orlhac F, et al. Tumor texture analysis in 18F-FDG PET: relationships between texture parameters, histogram indices, standardized uptake values, metabolic volumes, and total lesion glycolysis. J Nucl Med. 2014;55(3):414–22.
Polanec S, et al. Head-to-head comparison of PI-RADS v2 and PI-RADS v1. Eur J Radiol. 2016;85(6):1125–31.
Eiber M, et al. Prostate cancer molecular imaging standardized evaluation (PROMISE): proposed miTNM classification for the interpretation of PSMA-ligand PET/CT. J Nucl Med. 2018;59(3):469–78.
Zwanenburg A, et al. The image biomarker standardization initiative: standardized quantitative radiomics for high-throughput image-based phenotyping. Radiology. 2020;295(2):328.
Urraro F, et al. MRI Radiomics in prostate cancer: a reliability study. Front Oncol. 2021;5354.
Anconina R, et al. Combined 18F-FDG PET/CT radiomics and sarcopenia score in predicting relapse-free survival and overall survival in patients with esophagogastric cancer. Clin Nucl Med 2022;10.1097.
Peterson RA, Peterson MRA. Package ‘bestNormalize’. Normalizing transformation functions. R package version, 2020. 1.
Ankerst DP, et al. A contemporary prostate biopsy risk calculator based on multiple heterogeneous cohorts. Eur Urol. 2018;74(2):197–203.
Alqahtani S, et al. Prediction of prostate cancer Gleason score upgrading from biopsy to radical prostatectomy using pre-biopsy multiparametric MRI PIRADS scoring system. Sci Rep. 2020;10(1):1–9.
Demirci E, et al. Can SUVmax values of Ga-68-PSMA PET/CT scan predict the clinically significant prostate cancer? Nucl Med Commun. 2019;40(1):86.
Domachevsky L, et al. Comparison between pelvic PSMA-PET/MR and whole-body PSMA-PET/CT for the initial evaluation of prostate cancer: a proof of concept study. Eur Radiol. 2020;30(1):328–36.
Park SY, et al. Gallium 68 PSMA-11 PET/MR imaging in patients with intermediate-or high-risk prostate cancer. Radiology. 2018;288(2):495–505.
Ferraro DA, et al. Diagnostic performance of 68Ga-PSMA-11 PET/MRI-guided biopsy in patients with suspected prostate cancer: a prospective single-center study. Eur J Nucl Med Mol Imaging. 2021;48(10):3315–24.
Hofman MS, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. The Lancet. 2020;395(10231):1208–16.
Metser U, et al. Detection of clinically significant prostate cancer with 18F-DCFPyL PET/multiparametric MR. Eur J Nucl Med Mol Imaging. 2021;48(11):3702–11.
Freitag MT, et al. Local recurrence of prostate cancer after radical prostatectomy is at risk to be missed in 68Ga-PSMA-11-PET of PET/CT and PET/MRI: comparison with mpMRI integrated in simultaneous PET/MRI. Eur J Nucl Med Mol Imaging. 2017;44(5):776–87.
Kranzbühler B, et al. Clinical performance of 68Ga-PSMA-11 PET/MRI for the detection of recurrent prostate cancer following radical prostatectomy. Eur J Nucl Med Mol Imaging. 2018;45(1):20–30.
Hicks RM, et al. Diagnostic accuracy of 68Ga-PSMA-11 PET/MRI compared with multiparametric MRI in the detection of prostate cancer. Radiology. 2018;289(3):730–7.
Scheltema MJ, et al. Diagnostic accuracy of 68Ga-prostate-specific membrane antigen (PSMA) positron-emission tomography (PET) and multiparametric (mp) MRI to detect intermediate-grade intra-prostatic prostate cancer using whole-mount pathology: impact of the addition of 68Ga-PSMA PET to mpMRI. BJU Int. 2019;124(S1):42–9.
Burger IA, et al. 68Ga-PSMA-11 PET/MR detects local recurrence occult on mpMRI in prostate cancer patients after HIFU. J Nucl Med. 2019;60(8):1118–23.
Hillier SM, et al. Preclinical evaluation of novel glutamate-urea-lysine analogues that target prostate-specific membrane antigen as molecular imaging pharmaceuticals for prostate cancer. Can Res. 2009;69(17):6932–40.
Bonekamp D, et al. Radiomic machine learning for characterization of prostate lesions with MRI: comparison to ADC values. Radiology. 2018;289(1):128–37.
Gong L, et al. Noninvasive prediction of high-grade prostate cancer via biparametric MRI radiomics. J Magn Reson Imaging. 2020;52(4):1102–9.
Papp L, et al. Supervised machine learning enables non-invasive lesion characterization in primary prostate cancer with [68Ga] Ga-PSMA-11 PET/MRI. Eur J Nucl Med Mol Imaging. 2021;48(6):1795–805.
Feliciani G, et al. Radiomics Analysis on [68Ga] Ga-PSMA-11 PET and MRI-ADC for the Prediction of Prostate Cancer ISUP Grades: Preliminary Results of the BIOPSTAGE Trial. Cancers. 2022;14(8):1888.
Funding
Study supported by Toronto General Toronto Western Hospital Foundation (NCT03535831) and Mount Sinai Hospital-University Health Network Academic Medical Organization Innovation Fund 2017–2019 (NCT 03149861); Toronto, ON, Canada.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation and data collection were performed by Adriano Basso Dias and Seyed Ali Mirshahvalad. Statistical analysis was performed by Lisa Avery. The first draft of the manuscript was written by Adriano Basso Dias, Seyed Ali Mirshahvalad, and Patrick Veit-Haibach and all authors commented on previous versions of the manuscript. Supervision was performed by Patrick Veit-Haibach. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
This was an ethics review board-approved, retrospective analysis of two prospective clinical trials (ClinicalTrials.gov Identifiers: NCT03535831 and NCT03149861).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Competing interests
Alejandro Berlin: Participation on a DataSafety Monitoring Board or Advisory Board from Princess Margaret Cancer Center—University Health Network. Ur Metser: Consulting fees from POINT Biopharm; chair, Ontario PET Steering Committee and Ontario Health-Cancer Care Ontario. Patrick Veit-Haibach: Grants from Siemens Healthineers; support for attending meetings and/or travel from Siemens Healthineers; PET Center of Excellence committee of the Society of Nuclear Medicine member. The other authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Oncology - Genitourinary.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Basso Dias, A., Mirshahvalad, S.A., Ortega, C. et al. The role of [18F]-DCFPyL PET/MRI radiomics for pathological grade group prediction in prostate cancer. Eur J Nucl Med Mol Imaging 50, 2167–2176 (2023). https://doi.org/10.1007/s00259-023-06136-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-023-06136-0