Abstract
Purpose
During lung cancer surgery, it is very important to define tumor boundaries and determine the surgical margin distance. In previous research, systemically application of fluorescent probes can help medical professionals determine the boundaries of tumors and find small tumors and metastases, thereby improving the accuracy of surgical resection. However, there are very few safe and effective probes that can be applied to clinical trials up to now, which limits the clinical application of fluorescence imaging. Here we developed a new technology that can quickly identify the tumor area in the resected lung tissue during the operation and distinguish the tumor boundary and metastatic lymph nodes.
Experimental design
For animal studies, a PDX model of lung cancer was established. The tumors, lungs, and peritumoral muscle tissues of tumor-bearing mice were surgically removed and incubated with a probe targeting epidermal growth factor receptor (EGFR) for 20 min, and then imaged by a closed-field near-infrared two-zone (NIR-II) fluorescence imaging system. For clinical samples, ten surgically removed lung tissues and 60 lymph nodes from 10 lung cancer patients undergoing radical resection were incubated with the targeting probe immediately after intraoperative resection and imaged to identify the tumor area and distinguish the tumor boundary and metastatic lymph nodes. The accuracy of fluorescence imaging was confirmed by HE staining and immunohistochemistry.
Results
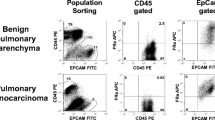
The ex vivo animal imaging experiments showed a fluorescence enhancement of tumor tissue. For clinical samples, our results showed that this new technology yielded more than 85.7% sensitivity and 100% specificity in identifying the tumor area in the resected lung tissue. The average fluorescence tumor-to-background ratio was 2.5 ± 1.3. Furthermore, we also used this technique to image metastatic lymph nodes intraoperatively and showed that metastatic lymph nodes have brighter fluorescence than normal lymph nodes, as the average fluorescence tumor-to-background signal ratio was 2.7 ± 1.1. Calculations on the results of the fluorescence signal in relation to the number of metastatic lymph nodes yielded values of 77.8% for sensitivity and 92.1% for specificity. We expect this new technology to be a useful diagnostic tool for rapid intraoperative pathological detection and margin determination.
Conclusions
By using fluorescently labeled anti-human EGFR recombinant antibody scFv fragment to incubate freshly isolated tissues during surgery, the probes can quickly accumulate in lung cancer tissues, which can be used to quickly identify tumor areas in the resected lung tissues and distinguish tumor boundaries and find metastases in lymph nodes. This technology is expected to be used for rapid intraoperative pathological detection and margin determination.







Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Data availability
The data generated or analyzed during this study are included in the manuscript and the supplementary information files.
References
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. https://doi.org/10.3322/caac.21708.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. https://doi.org/10.3322/caac.21660.
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7–33. https://doi.org/10.3322/caac.21654.
Hui Z, Men Y, Hu C, Kang J, Sun X, Bi N, et al. Effect of postoperative radiotherapy for patients with pIIIA-N2 non–small cell lung cancer after complete resection and adjuvant chemotherapy: the phase 3 PORT-C randomized clinical trial. JAMA Oncol. 2021;7:1178–85. https://doi.org/10.1001/jamaoncol.2021.1910.
Chi C, Du Y, Ye J, Kou D, Qiu J, Wang J, et al. Intraoperative imaging-guided cancer surgery: from current fluorescence molecular imaging methods to future multi-modality imaging technology. Theranostics. 2014;4:1072–84. https://doi.org/10.7150/thno.9899.
Okusanya OT, Hess NR, Luketich JD, Sarkaria IS. Infrared intraoperative fluorescence imaging using indocyanine green in thoracic surgery. Eur J Cardiothorac Surg. 2018;53:512–8.
Kossatz S, Pirovano G, De Souza Franca PD, Strome AL, Sunny SP, Zanoni DK, et al. Validation of the use of a fluorescent PARP1 inhibitor for the detection of oral, oropharyngeal and oesophageal epithelial cancers. Nat Biomed Eng. 2020;4:272–85. https://doi.org/10.1038/s41551-020-0526-9.
Hernot S, van Manen L, Debie P, Mieog JSD, Vahrmeijer AL. Latest developments in molecular tracers for fluorescence image-guided cancer surgery. Lancet Oncol. 2019;20:e354–67. https://doi.org/10.1016/s1470-2045(19)30317-1.
Quan YH, Oh CH, Jung D, Lim J-Y, Choi BH, Rho J, et al. Evaluation of intraoperative near-infrared fluorescence visualization of the lung tumor margin with indocyanine green inhalation. JAMA Surg. 2020;155:732–40.
Kennedy GT, Azari FS, Bernstein E, Marfatia I, Din A, Kucharczuk JC, et al. Targeted intraoperative molecular imaging for localizing nonpalpable tumors and quantifying resection margin distances. JAMA Surg. 2021;156:1043–50. https://doi.org/10.1001/jamasurg.2021.3757.
Miyamoto H. Intraoperative pathology consultation during urological surgery: Impact on final margin status and pitfalls of frozen section diagnosis. Pathol Int. 2021;71:567–80. https://doi.org/10.1111/pin.13132.
Shima T, Kinoshita T, Sasaki N, Uematsu M, Sugita Y, Shimizu R, et al. Feasibility of intraoperative diagnosis of lung adenocarcinoma in situ to avoid excessive resection. J Thorac Dis. 2021;13:1338–46. https://doi.org/10.21037/jtd-20-2710.
Yeh Y-C, Nitadori J-I, Kadota K, Yoshizawa A, Rekhtman N, Moreira AL, et al. Using frozen section to identify histological patterns in stage I lung adenocarcinoma of ≤3 cm: accuracy and interobserver agreement. Histopathology. 2015;66:922–38. https://doi.org/10.1111/his.12468.
Li F, Yang L, Zhao Y, Yuan L, Wang S, Mao Y. Intraoperative frozen section for identifying the invasion status of lung adenocarcinoma: a systematic review and meta-analysis. Int J Surg. 2019;72:175–84. https://doi.org/10.1016/j.ijsu.2019.10.047.
Rosenthal EL, Warram JM, de Boer E, Chung TK, Korb ML, Brandwein-Gensler M, et al. Safety and tumor specificity of cetuximab-IRDye800 for surgical navigation in head and neck cancer. Clin Cancer Res. 2015;21:3658–66. https://doi.org/10.1158/1078-0432.CCR-14-3284.
Lauwerends LJ, van Driel PB, de Jong RJB, Hardillo JA, Koljenovic S, Puppels G, et al. Real-time fluorescence imaging in intraoperative decision making for cancer surgery. Lancet Oncol. 2021;22:e186–95.
van Keulen S, Nishio N, Birkeland A, Fakurnejad S, Martin B, Forouzanfar T, et al. The sentinel margin: intraoperative ex vivo specimen mapping using relative fluorescence intensity. Clin Cancer Res. 2019;25:4656–62. https://doi.org/10.1158/1078-0432.CCR-19-0319.
Linssen MD, Ter Weele EJ, Allersma DP, Lub-de Hooge MN, van Dam GM, Jorritsma-Smit A, et al. Roadmap for the development and clinical translation of optical tracers cetuximab-800CW and trastuzumab-800CW. J Nucl Med. 2019;60:418–23. https://doi.org/10.2967/jnumed.118.216556.
Rosenthal EL, Moore LS, Tipirneni K, de Boer E, Stevens TM, Hartman YE, et al. Sensitivity and specificity of cetuximab-IRDye800CW to identify regional metastatic disease in head and neck cancer. Clin Cancer Res. 2017;23:4744–52. https://doi.org/10.1158/1078-0432.CCR-16-2968.
Gao RW, Teraphongphom N, de Boer E, van den Berg NS, Divi V, Kaplan MJ, et al. Safety of panitumumab-IRDye800CW and cetuximab-IRDye800CW for fluorescence-guided surgical navigation in head and neck cancers. Theranostics. 2018;8:2488–95. https://doi.org/10.7150/thno.24487.
Day KE, Sweeny L, Kulbersh B, Zinn KR, Rosenthal EL. Preclinical comparison of near-infrared-labeled cetuximab and panitumumab for optical imaging of head and neck squamous cell carcinoma. Mol Imag Biol. 2013;15:722–9. https://doi.org/10.1007/s11307-013-0652-9.
Vonk J, Voskuil FJ, de Wit JG, Heeman WT, Nagengast WB, van Dam GM, et al. Fluorescence grid analysis for the evaluation of piecemeal surgery in sinonasal inverted papilloma: a proof-of-concept study. Eur J Nucl Med Mol Imaging. 2022;49:1640–9. https://doi.org/10.1007/s00259-021-05567-x.
Quan YH, Bao K, Kim K, Wang H, Yokomizo S, Park GK, et al. Abstract 5975: Dual-channel near-infrared fluorescence imaging for simultaneous identification of lung cancer and intersegmental plane. Cancer Res. 2022;82:5975. https://doi.org/10.1158/1538-7445.AM2022-5975.
Yang J, He S, Hu Z, Zhang Z, Cao C, Cheng Z, et al. In vivo multifunctional fluorescence imaging using liposome-coated lanthanide nanoparticles in near-infrared-II/IIa/IIb windows. Nano Today. 2021;38:101120. https://doi.org/10.1016/j.nantod.2021.101120.
Chang B, Li D, Ren Y, Qu C, Shi X, Liu R, et al. A phosphorescent probe for in vivo imaging in the second near-infrared window. Nat Biomed Eng. 2022;6:629–39. https://doi.org/10.1038/s41551-021-00773-2.
Lei Z, Zhang F. Molecular engineering of NIR-II fluorophores for improved biomedical detection. Angew Chem Int Ed. 2021;60:16294–308. https://doi.org/10.1002/anie.202007040.
Tao W, Farokhzad OC. Theranostic nanomedicine in the NIR-II window: classification, fabrication, and biomedical applications. Chem Rev. 2022;122:5405–7. https://doi.org/10.1021/acs.chemrev.2c00089.
Hu Z, Fang C, Li B, Zhang Z, Cao C, Cai M, et al. First-in-human liver-tumour surgery guided by multispectral fluorescence imaging in the visible and near-infrared-I/II windows. Nat Biomed Eng. 2020;4:259–71. https://doi.org/10.1038/s41551-019-0494-0.
Eatz TA, Eichberg DG, Lu VM, Di L, Komotar RJ, Ivan ME. Intraoperative 5-ALA fluorescence-guided resection of high-grade glioma leads to greater extent of resection with better outcomes: a systematic review. J Neurooncol. 2022;156:233–56. https://doi.org/10.1007/s11060-021-03901-9.
Duan H, Pang Y, Zhao C, Zhou T, Sun C, Hou M, et al. A novel, minimally invasive technique to establish the animal model of spinal cord injury. Ann Transl Med. 2021;9:881. https://doi.org/10.21037/atm-21-2063.
Patrunov Y, Sencha AN, Sencha E, Penyaeva E. Ultrasound of regional lymph nodes in breast cancer. In: Sukhikh GT, Sencha AN, editors. Multiparametric ultrasound diagnosis of breast diseases. Cham: Springer International Publishing; 2018. p. 265–84.
Yinhao, Pan Ningbo, Chen Liangjian, Liu Chengbo, Liu Zhiqiang, Xu Jianhui, Zhang (2021) Recovery of photoacoustic images based on accurate ultrasound positioning. Visual Computing for Industry Biomedicine and Art 4(1):7. https://doi.org/10.1186/s42492-021-00072-2
Lubner MG, Mankowski Gettle L, Kim DH, Ziemlewicz TJ, Dahiya N, Pickhardt P. Diagnostic and procedural intraoperative ultrasound: technique, tips and tricks for optimizing results. Br J Radiol. 2021;94:20201406. https://doi.org/10.1259/bjr.20201406.
Fuentes AM, Ansari D, Burch TG, Mehta AI. Use of intraoperative MRI for resection of intracranial tumors: a nationwide analysis of short-term outcomes. J Clin Neurosci. 2022;99:152–7. https://doi.org/10.1016/j.jocn.2022.03.005.
Golub D, Hyde J, Dogra S, Nicholson J, Kirkwood KA, Gohel P, et al. Intraoperative MRI versus 5-ALA in high-grade glioma resection: a network meta-analysis. J Neurosurg JNS. 2021;134:484–98. https://doi.org/10.3171/2019.12.JNS191203.
Rogers CM, Jones PS, Weinberg JS. Intraoperative MRI for brain tumors. J Neurooncol. 2021;151:479–90. https://doi.org/10.1007/s11060-020-03667-6.
Qiu Y, Zhang Y, Li M, Chen G, Fan C, Cui K, et al. Intraoperative detection and eradication of residual microtumors with gap-enhanced Raman tags. ACS Nano. 2018;12:7974–85. https://doi.org/10.1021/acsnano.8b02681.
Cui K, Zhang Y, Chen G, Cui Y, Wu W, Zhao N, et al. Molecular regulation of polymeric Raman probes for ultrasensitive microtumor diagnosis and noninvasive microvessel imaging. Small. 2022;18:2106925. https://doi.org/10.1002/smll.202106925.
Jiang C, Wang Y, Song W, Lu L. Delineating the tumor margin with intraoperative surface-enhanced Raman spectroscopy. Anal Bioanal Chem. 2019;411:3993–4006. https://doi.org/10.1007/s00216-019-01577-9.
Jermyn M, Mok K, Mercier J, Desroches J, Pichette J, Saint-Arnaud K, et al. Intraoperative brain cancer detection with Raman spectroscopy in humans. Sci Translat Med. 2015;7:274ra19. https://doi.org/10.1126/scitranslmed.aaa2384.
Acknowledgements
This work was supported in part by the National Natural Science Foundation of China (grants no. 62027901), the Fundamental Research Funds for the Central Universities (grant no. JKF-YG-22-B005), the National Key Research and Development Program of China (under Grants 2017YFA0205200, 2016YFC0103803, and 2017YFA0700401), the National Natural Science Foundation of China (grants nos. 81227901, 81930053, 81827808, 81527805, 81971198, 81671851, 82003316, and 92059203), China Postdoctoral Science Foundation (grant nos. 2020M680301 and 2019TQ0018), Chinese Academy of Sciences Youth Innovation Promotion Association (grant no. 2018167), CAS Youth Interdisciplinary Team (JCTD-2021-08), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA16021200), Chinese Academy of Sciences Key Technology Talent Program, Project of High-Level Talents Team Introduction in Zhuhai City (Zhuhai HLHPTP201703), Beijing Natural Science Foundation (grant nos. 7214266, JQ19027), and Research and Development Fund of Peking University People’s Hospital (grant no. RS2020-05). The authors would like to acknowledge the instrumental technical support of the Multimodal Biomedical Imaging Experimental Platform, Institute of Automation, Chinese Academy of Sciences.
Author information
Authors and Affiliations
Contributions
Changjian Li synthesized the probe. Changjian Li and Jiahui Mi performed the experiments. Changjian Li and Yueqi Wang analyzed the data and wrote the manuscript. Jie Tian, Zhenhua Hu, and Jian Zhou supervised the designation of the experiments. Zeyu Zhang and Xiaoyong Guo assisted with data analysis.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
In human subjects, the study was approved by the Institutional Review Board at the Peking University People’s Hospital (Permit Number: 2021PHB315-001). In animal subjects, all experiments were performed according to the guidelines of the Institutional Animal Care and Use Committee of Beijing Municipal Science & Technology Commission (Permit Number: 2020–0049).
Consent for publication
All the co-authors approved the manuscript and agreed with submission to your esteemed journal.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Theragnostic.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, C., Mi, J., Wang, Y. et al. New and effective EGFR-targeted fluorescence imaging technology for intraoperative rapid determination of lung cancer in freshly isolated tissue. Eur J Nucl Med Mol Imaging 50, 494–507 (2023). https://doi.org/10.1007/s00259-022-05975-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-022-05975-7