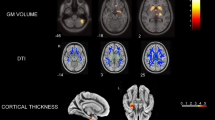
Abstract
Purpose
Progressive supranuclear palsy (PSP) is primary 4-repeat tauopathy. Evidence spanning from imaging studies indicate aberrant connectivity in PSPs. Our goal was to assess functional connectivity network alterations in PSP patients and the potential link between regional tau-burden and network-level functional connectivity using the next-generation tau PET tracer [18F]PI-2620 and resting-state functional MRI (fMRI).
Material and methods
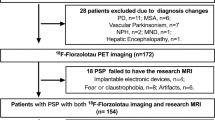
Twenty-four probable PSP patients (70.9 ± 6.9 years, 13 female), including 14 Richardson syndrome (RS) and 10 non-RS phenotypes, underwent [18F]PI-2620 PET/MRI imaging. Distribution volume ratios (DVRs) were estimated using non-invasive pharmacokinetic modeling. Resting-state fMRI was also acquired in these patients as well as in thirteen older non-AD MCI reference group (64 ± 9 years, 4 female). The functional network was constructed using 141 by 141 region-to-region functional connectivity metrics (RRC) and network-based statistic was carried out (connection threshold p < 0.001, cluster threshold pFDR < 0.05).
Results
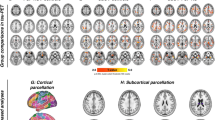
In total, 9870 functional connections were analyzed. PSPs compared to aged non-AD MCI reference group expressed aberrant connectivity evidenced by the significant NBS network consisting of 89 ROIs and 118 connections among them (NBS mass 4226, pFDR < 0.05). Tau load in the right globus pallidus externus (GPe) and left dentate nucleus (DN) showed significant effects on functional network connectivity. The network linked with increased tau load in the right GPe was associated with hyperconnectivity of low-range intra-opercular connections (NBS mass 356, pFDR < 0.05), while the network linked with increased tau load in the left cerebellar DN was associated with cerebellar hyperconnectivity and cortico-cerebellar hypoconnectivity (NBS mass 517, pFDR < 0.05).
Conclusions
PSP patients show altered functional connectivity. Network incorporating deep gray matter structures demonstrate hypoconnectivity, cerebellum hyperconnectivity, while cortico-cortical connections show variable changes. Tau load in the right GPe and left DN is associated with functional networks which strengthen low-scale intra-opercular and intra-cerebellar connections and weaken opercular-cerebellar connections. These findings support the concept of tau load-dependent functional network changes in PSP, by that providing evidence for downstream effects of neuropathology on brain functionality in this primary tauopathy.



Similar content being viewed by others
Data availability
Raw and preprocessed data will be made available upon reasonable request.
References
Goedert M, Spillantini MG. Pathogenesis of the tauopathies. J Mol Neurosci: MN. 2011;45:425–31.
VandeVrede L, Ljubenkov PA, Rojas JC, et al. Four-repeat tauopathies: current management and future treatments. Neurotherapeutics. 2020;17:1563–1581. https://doi.org/10.1007/s13311-020-00888-5
Williams DR, Lees AJ. Progressive supranuclear palsy: clinicopathological concepts and diagnostic challenges. Lancet Neurol. 2009;8:270–9.
Höglinger GU, Respondek G, Stamelou M, Kurz C, Josephs KA, Lang AE, et al. Clinical diagnosis of progressive supranuclear palsy: the movement disorder society criteria. Mov Disord: Off J Mov Disord Soc. 2017;32:853–64.
Whitwell JL, Höglinger GU, Antonini A, Bordelon Y, Boxer AL, Colosimo C, et al. Radiological biomarkers for diagnosis in PSP: where are we and where do we need to be? Mov Disord: Off J Mov Disord Soc. 2017;32:955–71.
Albrecht F, Bisenius S, Neumann J, Whitwell J, Schroeter ML. Atrophy in midbrain & cerebral/cerebellar pedunculi is characteristic for progressive supranuclear palsy – a double-validation whole-brain meta-analysis. NeuroImage: Clinical. 2019;22:101722.
Beyer L, Meyer-Wilmes J, Schönecker S, Schnabel J, Brendel E, Prix C, et al. Clinical routine FDG-PET imaging of suspected progressive supranuclear palsy and corticobasal degeneration: a gatekeeper for subsequent tau-PET imaging? Front Neurol. 2018;9:483.
Zhao P, Zhang B, Gao S, Li X. Clinical, MRI and 18F-FDG-PET/CT analysis of progressive supranuclear palsy. J Clin Neurosci. 2020;80:318–23.
Bharti K, Bologna M, Upadhyay N, Piattella MC, Suppa A, Petsas N, et al. Abnormal resting-state functional connectivity in progressive supranuclear palsy and corticobasal syndrome. Front Neurol. 2017;8:1247.
Piattella MC, Tona F, Bologna M, Sbardella E, Formica A, Petsas N, et al. Disrupted resting-state functional connectivity in progressive supranuclear palsy. Am J Neuroradiol. 2015;36:915–21.
Whitwell JL, Avula R, Master A, Vemuri P, Senjem ML, Jones DT, et al. Disrupted thalamocortical connectivity in PSP: a resting-state fMRI, DTI, and VBM study. Parkinsonism Relat Disord. 2011;17:599–605.
Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10:186–98.
Kovacs GG, Lukic MJ, Irwin DJ, Arzberger T, Respondek G, Lee EB, et al. Distribution patterns of tau pathology in progressive supranuclear palsy. Acta Neuropathol. 2020;140:99–119 (Springer Berlin Heidelberg).
Smith SM, Vidaurre D, Beckmann CF, Glasser MF, Jenkinson M, Miller KL, et al. Functional connectomics from resting-state fMRI. Trends Cogn Sci. 2013;17:666–82.
Schöll M, Maass A, Mattsson N, Ashton NJ, Blennow K, Zetterberg H, et al. Biomarkers for tau pathology. Mol Cell Neurosci. 2019;97:18–33.
Whitwell JL. Tau imaging in parkinsonism: what have we learned so far? Mov Disord Clin Pract. 2018;5:118–30.
Kroth H, Oden F, Molette J, Schieferstein H, Capotosti F, Mueller A, et al. Discovery and preclinical characterization of [18F]PI-2620, a next-generation tau PET tracer for the assessment of tau pathology in Alzheimer’s disease and other tauopathies. Eur J Nucl Med Mol Imaging. 2019;46:2178–89.
Brendel M, Barthel H, van Eimeren T, et al. Assessment of 18F-PI-2620 as a Biomarker in Progressive Supranuclear Palsy. JAMA Neurol. 2020;77(11):1408–1419. https://doi.org/10.1001/jamaneurol.2020.2526.
Golbe LI, Ohman-Strickland PA. A clinical rating scale for progressive supranuclear palsy. Brain. 2007;130:1552–65.
Schwab RS, England AC. Projection technique for evaluating surgery in Parkinson’s disease. In: Billingham FH, Donaldson MC, editors. Third symposium on Parkinson’s disease. Edinburgh: Churchill Livingstone; 1969. p. 152–7.
Guy W. ECDEU assessment manual for psychopharmacology, Revised. US Department of Health, Education, and Welfare Publication (ADM). Rockville: National Institute of Mental Health. 1976. p. 76–338.
Ladefoged CN, Law I, Anazodo U, St Lawrence K, Izquierdo-Garcia D, Catana C, et al. A multi-centre evaluation of eleven clinically feasible brain PET/MRI attenuation correction techniques using a large cohort of patients. Neuroimage. 2017;147:346–59.
Makris N, Goldstein JM, Kennedy D, Hodge SM, Caviness VS, Faraone SV, Tsuang MT, Seidman LJ. Decreased volume of left and total anterior insular lobule in schizophrenia. Schizophr Res. 2006;83(2–3):155–71.
Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Étard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89.
Keuken MC, Forstmann BU. A probabilistic atlas of the basal ganglia using 7 T MRI. Data Brief. 2015;4:577–82.
Diedrichsen J, Balsters JH, Flavell J, Cussans E, Ramnani N. A probabilistic MR atlas of the human cerebellum. NeuroImage. 2009;46:39–46 (Elsevier B.V.).
Ichise M, Liow J-S, Lu J-Q, Takano A, Model K, Toyama H, et al. Linearized reference tissue parametric imaging methods: application to [11C]DASB positron emission tomography studies of the serotonin transporter in human brain. J Cereb Blood Flow Metab. 2003;23:1096–112.
Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2:125–41.
Zalesky A, Fornito A, Bullmore ET. Network-based statistic: identifying differences in brain networks. NeuroImage. 2010;53:1197–207 (Elsevier Inc.).
Gardner RC, Boxer AL, Trujillo A, Mirsky JB, Guo CC, Gennatas ED, et al. Intrinsic connectivity network disruption in progressive supranuclear palsy. Ann Neurol. 2013;73:603–16.
Rosskopf J, Gorges M, Müller H-P, Lulé D, Uttner I, Ludolph AC, et al. Intrinsic functional connectivity alterations in progressive supranuclear palsy: differential effects in frontal cortex, motor, and midbrain networks. Mov Disord: Off J Mov Disord Soc. 2017;32:1006–15.
Warren JD, Rohrer JD, Schott JM, Fox NC, Hardy J, Rossor MN. Molecular nexopathies: a new paradigm of neurodegenerative disease. Trends Neurosci. 2013;36:561–9.
Conway BR. The organization and operation of inferior temporal cortex. Annu Rev Vis Sci. 2018;4:381–402.
Abos A, Segura B, Baggio HC, Campabadal A, Uribe C, Garrido A, et al. Disrupted structural connectivity of fronto-deep gray matter pathways in progressive supranuclear palsy. Neuroimage Clin. 2019;23:101899.
Piao Y-S, Hayashi S, Wakabayashi K, Kakita A, Aida I, Yamada M, et al. Cerebellar cortical tau pathology in progressive supranuclear palsy and corticobasal degeneration. Acta Neuropathol. 2002;103:469–74.
Voogd J. The human cerebellum. J Chem Neuroanat. 2003;26:243–52.
Cope TE, Rittman T, Borchert RJ, Jones PS, Vatansever D, Allinson K, et al. Tau burden and the functional connectome in Alzheimer’s disease and progressive supranuclear palsy. Brain. 2018;141:550–67.
Murugan NA, Chiotis K, Rodriguez-Vieitez E, et al. Cross-interaction of tau PET tracers with monoamine oxidase B: evidence from in silico modelling and in vivo imaging. Eur J Nucl Med Mol Imaging. 2019;46:1369–1382. https://doi.org/10.1007/s00259-019-04305-8
Barthel H. First tau PET tracer approved: toward accurate in vivo diagnosis of Alzheimer disease. J Nucl Med. 2020;61:1409–10.
Galvan A, Devergnas A, Wichmann T. Alterations in neuronal activity in basal ganglia-thalamocortical circuits in the parkinsonian state. Front. Neuroanat. 2015;9:5. https://doi.org/10.3389/fnana.2015.00005.
Rytty R, Nikkinen J, Paavola L, Abou Elseoud A, Moilanen V, Visuri A, Tervonen O, Renton AE, Traynor BJ, Kiviniemi V, Remes AM. GroupICA dual regression analysis of resting state networks in a behavioral variant of frontotemporal dementia. Front Hum Neurosci. 2013;7:461. https://doi.org/10.3389/fnhum.2013.00461.
Acknowledgements
The study was presented during the European Association of Nuclear Medicine 34th Annual Congress (EANM’21): Featured Session: Molecular Imaging of Movement Disorders (OP-0590).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Conceptualization: G. Aghakhanyan, M. Rullmann, O. Sabri, H. Barthel; methodology: G. Aghakhanyan, M. Rullmann, O. Sabri, H. Barthel; formal analysis and investigation: G. Aghakhanyan, M. Rullmann; writing—original draft preparation: G. Aghakhanyan; writing—review and editing: M. Rullmann, Jost-Julian Rumpf, M. L. Schroeter, C. Scherlach, M. Patt, M. Brendel, N. Koglin, A. W. Stephens, J. Classen, K. T. Hoffmann, O. Sabri, H. Barthel; resources: Jost-Julian Rumpf, M. L. Schroeter, C. Scherlach, M. Patt, M. Brendel, N. Koglin, A. W. Stephens, J. Classen, K. T. Hoffmann, O. Sabri, H. Barthel; supervision: M. Rullmann, O. Sabri, H. Barthel. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The study was approved by the local Ethics Committee of the University of Leipzig.
Consent to participate
Informed consent was obtained from all participants.
Conflict of interest
Authors G. Aghakhanyan, M. Rullmann, M. Patt, J. Classen, and O. Sabri declare that they have no relevant financial conflicts of interests and nothing to disclose. Author J. J. Rumpf has received speaker and consultant honoraria from GE Healthcare. M. L. Schroeter has been supported by the German Research Foundation (DFG; SCHR 774/5–1) and eHealthSax Initiative of the Sächsische Aufbaubank (SAB). M. Brendel received speaker honoraria from Roche, GE Healthcare, and LMI and is an advisor of LMI. A. Stephens and N. Koglin are full-time employees of Life Molecular Imaging, GmbH. K. T. Hoffmann has received speaker and consultant honoraria from Bayer. H. Barthel received speaker honoraria from AAA/Novartis and reader honoraria from LMI.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Neurology – Movement disorders.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aghakhanyan, G., Rullmann, M., Rumpf, J. et al. Interplay of tau and functional network connectivity in progressive supranuclear palsy: a [18F]PI-2620 PET/MRI study. Eur J Nucl Med Mol Imaging 50, 103–114 (2022). https://doi.org/10.1007/s00259-022-05952-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-022-05952-0