Abstract
Purpose
Accurate assessment of residual disease of tumor and lymph nodes after neoadjuvant immunochemotherapy is crucial in the active surveillance for patients with pathological complete response (pCR) and the optimal extent of lymphadenectomy for patients with non-pCR. This post hoc analysis aimed to evaluate the performance of 18F-FDG PET/CT to predict the pathological response to neoadjuvant immunochemotherapy for esophageal squamous cell carcinoma (ESCC).
Methods
Fifty-eight resectable ESCC patients received two cycles of camrelizumab in combination with chemotherapy and were enrolled in the final analysis. The 18F-FDG PET/CT scans were acquired at baseline (scan-1) and after immunochemotherapy but prior to surgery (scan-2). Maximum standardized uptake value (SUVmax), mean standardized uptake value (SUVmean), tumor-to-blood pool SUVmax ratio (SUVTBR), metabolic tumor volume (MTV), and total lesion glycolysis (TLG) were evaluated for their association with the pathological response to immunochemotherapy.
Results
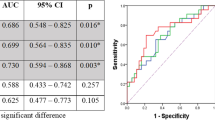
Nineteen patients (32.8%, 19/58) had pCR and thirty-nine patients (67.2%, 39/58) had non-pCR after two doses of camrelizumab and chemotherapy. At scan-2, the SUVmax, SUVmean, SUVTBR, TLG, and MTV were significantly lower in pCR than in non-pCR patients. Decrease in TLG and MTV between scan-2 and scan-1 of the same patient was significantly higher in the pCR than in the non-pCR group. In the receiver operating characteristic curve analysis, SUVmax, SUVmean, SUVTBR, TLG, and MTV in scan-2 showed excellent predictive value for the pCR of primary tumors. Furthermore, SUVmax in scan-2 were higher in positive lymph nodes than in negative ones, suggesting a high negative predictive ability (98.6%) with a cut-off value at 1.4.
Conclusion
The parameters of 18F-FDG PET/CT have the excellent performance for predicting pCR after the combined neoadjuvant immunochemotherapy in resectable ESCC.
Trial registration
ChiCTR2000028900. Registered on January 6, 2020.


Similar content being viewed by others
Abbreviations
- pCR:
-
Pathological complete response
- ESCC:
-
Esophageal squamous cell carcinoma
- SUVmax :
-
Maximum standardized uptake value
- SUVmean :
-
Mean standardized uptake value
- SUVTBR :
-
Tumor-to-blood pool SUVmax ratio
- MTV:
-
Metabolic tumor volume
- TLG:
-
Total lesion glycolysis
- nCRT:
-
Neoadjuvant chemoradiotherapy
- PD-1:
-
Programmed cell death 1
- DFS:
-
Disease-free survival
- OS:
-
Overall survival
- EC:
-
Esophageal cancer
- 18F-FDG:
-
Fluorine 18-fluorodeoxyglucose
- SAD:
-
Short axis diameter
- IQR:
-
Interquartile range
- ROC:
-
Receiver operating characteristic
- AUC:
-
Area under the ROC curve
- PPV:
-
Positive predictive value
- NPV:
-
Negative predictive value
- CI:
-
Confidence intervals
- LN:
-
Lymph node
References
Shapiro J, van Lanschot JJB, Hulshof M, van Hagen P, van Berge Henegouwen MI, Wijnhoven BPL, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16:1090–8. https://doi.org/10.1016/S1470-2045(15)00040-6.
Yang H, Liu H, Chen Y, Zhu C, Fang W, Yu Z, et al. Neoadjuvant chemoradiotherapy followed by surgery versus surgery alone for locally advanced squamous cell carcinoma of the esophagus (NEOCRTEC5010): a phase III multicenter, randomized, open-label clinical trial. J Clin Oncol : official journal of the American Society of Clinical Oncology. 2018;36:2796–803. https://doi.org/10.1200/jco.2018.79.1483.
Wang H, Tang H, Fang Y, Tan L, Yin J, Shen Y, et al. Morbidity and mortality of patients who underwent minimally invasive esophagectomy after neoadjuvant chemoradiotherapy vs neoadjuvant chemotherapy for locally advanced esophageal squamous cell carcinoma: a randomized clinical trial. JAMA Surg. 2021;156:444–51. https://doi.org/10.1001/jamasurg.2021.0133.
Kojima T, Shah MA, Muro K, Francois E, Adenis A, Hsu CH, et al. Randomized phase III KEYNOTE-181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J Clin Oncol. 2020;38:4138–48. https://doi.org/10.1200/JCO.20.01888.
Sun JM, Shen L, Shah MA, Enzinger P, Adenis A, Doi T, et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet. 2021;398:759–71. https://doi.org/10.1016/s0140-6736(21)01234-4.
Yang W, Xing X, Yeung S, Wang S, Chen W, Bao Y, et al. Neoadjuvant programmed cell death 1 blockade combined with chemotherapy for resectable esophageal squamous cell carcinoma. J Immunother Cancer. 2022;10. https://doi.org/10.1136/jitc-2021-003497.
Blum Murphy M, Xiao L, Patel V, Maru D, Correa A, G Amlashi F, et al. Pathological complete response in patients with esophageal cancer after the trimodality approach: the association with baseline variables and survival-The University of Texas MD Anderson Cancer Center experience. Cancer. 2017;123:4106–13. https://doi.org/10.1002/cncr.30953.
Donahue J, Nichols F, Li Z, Schomas D, Allen M, Cassivi S, et al. Complete pathologic response after neoadjuvant chemoradiotherapy for esophageal cancer is associated with enhanced survival. Ann Thorac Surg. 2009;87:392–8; discussion 8–9. https://doi.org/10.1016/j.athoracsur.2008.11.001.
van der Wilk BJ, Noordman BJ, Neijenhuis LKA, Nieboer D, Nieuwenhuijzen GAP, Sosef MN, et al. Active surveillance versus immediate surgery in clinically complete responders after neoadjuvant chemoradiotherapy for esophageal cancer: a multicenter propensity matched study. Ann Surg. 2021;274:1009–16. https://doi.org/10.1097/SLA.0000000000003636.
Hiranyatheb P, Osugi H. Radical lymphadenectomy in esophageal cancer: from the past to the present. Dis Esophagus. 2015;28:68–77. https://doi.org/10.1111/dote.12091.
Westerterp M, van Westreenen H, Reitsma J, Hoekstra O, Stoker J, Fockens P, et al. Esophageal cancer: CT, endoscopic US, and FDG PET for assessment of response to neoadjuvant therapy–systematic review. Radiology. 2005;236:841–51. https://doi.org/10.1148/radiol.2363041042.
Makino T, Yamasaki M, Tanaka K, Tatsumi M, Takiguchi S, Hatazawa J, et al. Importance of positron emission tomography for assessing the response of primary and metastatic lesions to induction treatments in T4 esophageal cancer. Surgery. 2017;162:836–45. https://doi.org/10.1016/j.surg.2017.06.007.
Schmidt T, Lordick F, Herrmann K, Ott K. Value of functional imaging by PET in esophageal cancer. J Natl Compr Canc Net : JNCCN. 2015;13:239–47. https://doi.org/10.6004/jnccn.2015.0030.
Sánchez-Izquierdo N, Perlaza P, Pagès M, Buxó E, Rios J, Rubello D, et al. Assessment of response to neoadjuvant chemoradiotherapy by 18F-FDG PET/CT in patients with locally advanced esophagogastric junction adenocarcinoma. Clin Nucl Med. 2020;45:38–43. https://doi.org/10.1097/rlu.0000000000002840.
Kukar M, Alnaji R, Jabi F, Platz T, Attwood K, Nava H, et al. Role of Repeat 18F-fluorodeoxyglucose positron emission tomography examination in predicting pathologic response following neoadjuvant chemoradiotherapy for esophageal adenocarcinoma. JAMA Surg. 2015;150:555–62. https://doi.org/10.1001/jamasurg.2014.3867.
Metser U, Rashidi F, Moshonov H, Wong R, Knox J, Guindi M, et al. (18)F-FDG-PET/CT in assessing response to neoadjuvant chemoradiotherapy for potentially resectable locally advanced esophageal cancer. Ann Nucl Med. 2014;28:295–303. https://doi.org/10.1007/s12149-014-0812-2.
Lheureux S, Denoyelle C, Ohashi PS, De Bono JS, Mottaghy FM. Molecularly targeted therapies in cancer: a guide for the nuclear medicine physician. Eur J Nucl Med Mol Imaging. 2017;44:41–54. https://doi.org/10.1007/s00259-017-3695-3.
Tao XL, Li N, Wu N, He J, Ying JM, Gao SG, et al. The efficiency of F-18-FDG PET-CT for predicting the major pathologic response to the neoadjuvant PD-1 blockade in resectable non-small cell lung cancer. Eur J Nucl Med Mol Imaging. 2020;47:1209–19. https://doi.org/10.1007/s00259-020-04711-3.
Gao SG, Li N, Gao SY, Xue Q, Ying JM, Wang SH, et al. Neoadjuvant PD-1 inhibitor (Sintilimab) in NSCLC. J Thorac Oncol. 2020;15:816–26. https://doi.org/10.1016/j.jtho.2020.01.017.
Rice TW, Ishwaran H, Ferguson MK, Blackstone EH, Goldstraw P. Cancer of the esophagus and esophagogastric junction: an eighth edition staging primer. J Thorac Oncol. 2017;12:36–42. https://doi.org/10.1016/j.jtho.2016.10.016.
de Gouw DJJM, Klarenbeek BR, Driessen M, Bouwense SAW, van Workum F, Futterer JJ, et al. Detecting pathological complete response in esophageal cancer after neoadjuvant therapy based on imaging techniques: a diagnostic systematic review and meta-analysis. J Thorac Oncol. 2019;14:1156–71. https://doi.org/10.1016/j.jtho.2019.04.004.
Dormann CF, Elith J, Bacher S, Buchmann C, Carl G, Carre G, et al. Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography. 2013;36:27–46. https://doi.org/10.1111/j.1600-0587.2012.07348.x.
Eyck BM, Onstenk BD, Noordman BJ, Nieboer D, Spaander MCW, Valkema R, et al. Accuracy of detecting residual disease after neoadjuvant chemoradiotherapy for esophageal cancer: a systematic review and meta-analysis. Ann Surg. 2020;271:245–56. https://doi.org/10.1097/SLA.0000000000003397.
Hamai Y, Hihara J, Emi M, Furukawa T, Yamakita I, Kurokawa T, et al. Ability of fluorine-18 fluorodeoxyglucose positron emission tomography to predict outcomes of neoadjuvant chemoradiotherapy followed by surgical treatment for esophageal squamous cell carcinoma. Ann Thorac Surg. 2016;102:1132–9. https://doi.org/10.1016/j.athoracsur.2016.04.011.
Dewan A, Sharma S, Dewan A, Khurana R, Gupta M, Pahuja A, et al. Impact on radiological and pathological response with neoadjuvant chemoradiation and its effect on survival in squamous cell carcinoma of thoracic esophagus. J Gastrointest Cancer. 2017;48:42–9. https://doi.org/10.1007/s12029-016-9870-0.
Noordman BJ, Spaander MCW, Valkema R, Wijnhoven BPL, van Berge Henegouwen MI, Shapiro J, et al. Detection of residual disease after neoadjuvant chemoradiotherapy for oesophageal cancer (preSANO): a prospective multicentre, diagnostic cohort study. Lancet Oncol. 2018;19:965–74. https://doi.org/10.1016/s1470-2045(18)30201-8.
Choi Y, Choi JY, Hong TH, Choi YL, Oh D, Woo SY, et al. Trimodality therapy for locally advanced esophageal squamous cell carcinoma: the role of volume-based PET/CT in patient management and prognostication. Eur J Nucl Med Mol Imaging. 2022;49:751–62. https://doi.org/10.1007/s00259-021-05487-w.
Bensch F, van der Veen EL, Lub-de Hooge MN, Jorritsma-Smit A, Boellaard R, Kok IC, et al. (89)Zr-atezolizumab imaging as a non-invasive approach to assess clinical response to PD-L1 blockade in cancer. Nat Med. 2018;24:1852–8. https://doi.org/10.1038/s41591-018-0255-8.
Niemeijer AN, Leung D, Huisman MC, Bahce I, Hoekstra OS, van Dongen G, et al. Whole body PD-1 and PD-L1 positron emission tomography in patients with non-small-cell lung cancer. Nat Commun. 2018;9:4664. https://doi.org/10.1038/s41467-018-07131-y.
Iravani A, Hicks RJ. Imaging the cancer immune environment and its response to pharmacologic intervention, Part 2: the role of novel PET agents. J Nucl Med. 2020;61:1553–9. https://doi.org/10.2967/jnumed.120.248823.
Acknowledgements
We thank the patients and their family members who gave their consent to participate in this study, as well as the research staffs at our hospital who carried out the study.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Ethics Committee of the First Affiliated Hospital of Sun Yat-sen University and with the principles of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards as previously reported (PMID: 35022193).
Consent to participate
Informed consent was obtained from all patients enrolled in the study.
Conflict of interest
The authors declare no conflict of interest relevant to this study. Outside the context of this study, Dr. Yeung had prior grant funding from Bristol-Myer Squibb, Assertio (previously DepoMed), and Bausch Health, and participated in an expert panel for Celgene, Inc.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xiaoyan Wang, Weixiong Yang, and Qian Zhou contributed equally as first authors.
This article is part of the Topical Collection on Oncology—Digestive tract.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, X., Yang, W., Zhou, Q. et al. The role of 18F-FDG PET/CT in predicting the pathological response to neoadjuvant PD-1 blockade in combination with chemotherapy for resectable esophageal squamous cell carcinoma. Eur J Nucl Med Mol Imaging 49, 4241–4251 (2022). https://doi.org/10.1007/s00259-022-05872-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-022-05872-z