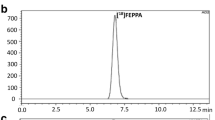
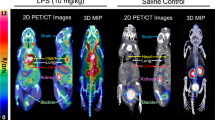
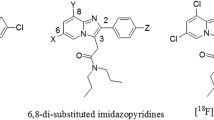
Abstract
Purpose
Hydrogen sulfide (H2S) plays important roles in brain pathophysiology. However, nuclear imaging probes for the in vivo detection of brain H2S in living animals have not been developed. Here, we report the first nuclear imaging probe that enables in vivo imaging of endogenous H2S in the brain of live mice.
Methods
Utilizing a bis(thiosemicarbazone) backbone, a fluorescent ATSM-FITC conjugate was synthesized. Its copper complex, Cu(ATSM-FITC) was thoroughly tested as a biosensor for H2S. The same ATSM-FITC ligand was quantitatively labeled with [64Cu]CuCl2 to obtain a radioactive [64Cu][Cu(ATSM-FITC)] imaging probe. Biodistribution and positron emission tomography (PET) imaging studies were performed in healthy mice and neuroinflammation models.
Results
The Cu(ATSM-FITC) complex reacts instantly with H2S to release CuS and becomes fluorescent. It showed excellent reactivity, sensitivity, and selectivity to H2S. Endogenous H2S levels in living cells were successfully detected by fluorescence microscopy. Exceptionally high brain uptake of [64Cu][Cu(ATSM-FITC)] (> 9% ID/g) was observed in biodistribution and PET imaging studies. Subtle changes in brain H2S concentrations in live mice were accurately detected by quantitative PET imaging. Due to its dual modality feature, increased H2S levels in neuroinflammation models were characterized at the subcellular level by fluorescence imaging and at the whole-body scale by PET imaging.
Conclusion
Our biosensor can be readily utilized to study brain H2S function in live animal models and shows great potential as a novel imaging agent for diagnosing brain diseases.
Graphical abstract







Similar content being viewed by others
Abbreviations
- AA:
-
Ascorbic acid
- AOAA:
-
Aminooxyacetic acid
- AP:
-
Anteroposterior
- BBB:
-
Blood-brain barrier
- CBS:
-
Cystathionine β-synthase
- CO:
-
Carbon monoxide
- CSE:
-
Cystathionine γ-lyase
- Cys:
-
L-cysteine
- DMSO:
-
Dimethyl sulfoxide
- DTT:
-
Dithiothreitol
- DV:
-
Dorsoventral
- FBS:
-
Fetal bovine serum
- FITC-NCS:
-
Fluorescein isothiocyanate
- GSH:
-
Glutathione
- H2O2 :
-
Hydrogen peroxide
- H2S:
-
Hydrogen sulfide
- Hcys:
-
L-homocysteine
- HEK293:
-
Human embryonic kidney 293 cell
- HeLa:
-
Human cervical cancer cell
- HepG2:
-
Human liver cancer cell
- HRMS:
-
High-resolution mass spectrometry
- LPS:
-
Lipopolysaccharide
- LOD:
-
Limit of detection
- MIP:
-
Maximum intensity projection
- ML:
-
Mediolateral
- NO:
-
Nitric oxide
- ONOO− :
-
Peroxynitrite
- PBS:
-
Phosphate-buffered saline
- PET:
-
Positron emission tomography
- PPG:
-
DL-propargylglycine
- U87MG:
-
Human brain glioblastoma cell
- 2-ME:
-
2-Mercaptoethanol
References
Abe K, Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci. 1996;16:1066–71. https://doi.org/10.1523/JNEUROSCI.16-03-01066.1996.
Cao X, Ding L, Xie ZZ, Yang Y, Whiteman M, Moore PK, et al. A review of hydrogen sulfide synthesis, metabolism, and measurement: is modulation of hydrogen sulfide a novel therapeutic for cancer? Antioxid Redox Signal. 2019;31:1–38. https://doi.org/10.1089/ars.2017.7058.
Nagpure BV, Bian JS. Brain, learning, and memory: role of H2S in neurodegenerative diseases. Handb Exp Pharmacol. 2015;230:193–215. https://doi.org/10.1007/978-3-319-18144-8_10.
Gong QH, Shi XR, Hong ZY, Pan LL, Liu XH, Zhu YZ. A new hope for neurodegeneration: possible role of hydrogen sulfide. J Alzheimers Dis. 2011;24(Suppl 2):173–82. https://doi.org/10.3233/JAD-2011-110128.
Zhang JY, Ding YP, Wang Z, Kong Y, Gao R, Chen G. Hydrogen sulfide therapy in brain diseases: from bench to bedside. Med Gas Res. 2017;7:113–9. https://doi.org/10.4103/2045-9912.208517.
Bae SK, Heo CH, Choi DJ, Sen D, Joe EH, Cho BR, et al. A ratiometric two-photon fluorescent probe reveals reduction in mitochondrial H2S production in Parkinson’s disease gene knockout astrocytes. J Am Chem Soc. 2013;135:9915–23. https://doi.org/10.1021/ja404004v.
Giovinazzo D, Bursac B, Sbodio JI, Nalluru S, Vignane T, Snowman AM, et al. Hydrogen sulfide is neuroprotective in Alzheimer’s disease by sulfhydrating GSK3b and inhibiting Tau hyperphosphorylation. Proc Natl Acad Sci USA. 2021;118:e2017225118. https://doi.org/10.1073/pnas.2017225118.
Zhang X, Bian JS. Hydrogen sulfide: a neuromodulator and neuroprotectant in the central nervous system. ACS Chem Neurosci. 2014;5:876–83. https://doi.org/10.1021/cn500185g.
Wallace JL, Wang R. Hydrogen sulfide-based therapeutics: exploiting a unique but ubiquitous gasotransmitter. Nat Rev Drug Discov. 2015;14:329–45. https://doi.org/10.1038/nrd4433.
Kang JM, Li Z, Organ CL, Park CM, Yang CT, Pacheco A, et al. pH-Controlled hydrogen sulfide release for myocardial ischemia-reperfusion injury. J Am Chem Soc. 2016;138:6336–9. https://doi.org/10.1021/jacs.6b01373.
Ibrahim H, Serag A, Farag MA. Emerging analytical tools for the detection of the third gasotransmitter H2S, a comprehensive review. J Adv Res. 2021;27:137–53. https://doi.org/10.1016/j.jare.2020.05.018.
Lippert AR, New EJ, Chang CJ. Reaction-based fluorescent probes for selective imaging of hydrogen sulfide in living cells. J Am Chem Soc. 2011;133:10078–80. https://doi.org/10.1021/ja203661j.
Sasakura K, Hanaoka K, Shibuya N, Mikami Y, Kimura Y, Komatsu T, et al. Development of a highly selective fluorescence probe for hydrogen sulfide. J Am Chem Soc. 2011;133:18003–5. https://doi.org/10.1021/ja207851s.
Lin VS, Lippert AR, Chang CJ. Cell-trappable fluorescent probes for endogenous hydrogen sulfide signaling and imaging H2O2-dependent H2S production. Proc Natl Acad Sci U S A. 2013;110:7131–5. https://doi.org/10.1073/pnas.1302193110.
Lin VS, Chen W, Xian M, Chang CJ. Chemical probes for molecular imaging and detection of hydrogen sulfide and reactive sulfur species in biological systems. Chem Soc Rev. 2015;44:4596–618. https://doi.org/10.1039/c4cs00298a.
Hammers MD, Taormina MJ, Cerda MM, Montoya LA, Seidenkranz DT, Parthasarathy R, et al. A bright fluorescent probe for H2S enables analyte-responsive, 3D imaging in live Zebrafish using light sheet fluorescence microscopy. J Am Chem Soc. 2015;137:10216–23. https://doi.org/10.1021/jacs.5b04196.
Shimamoto K, Hanaoka K. Fluorescent probes for hydrogen sulfide (H2S) and sulfane sulfur and their applications to biological studies. Nitric Oxide. 2015;46:72–9. https://doi.org/10.1016/j.niox.2014.11.008.
Pak YL, Li J, Ko KC, Kim G, Lee JY, Yoon J. Mitochondria-targeted reaction-based fluorescent probe for hydrogen sulfide. Anal Chem. 2016;88:5476–81. https://doi.org/10.1021/acs.analchem.6b00956.
Peng JJ, Teoh CL, Zeng X, Samanta A, Wang L, Xu W, et al. Development of a highly selective, sensitive, and fast response upconversion luminescent platform for hydrogen sulfide detection. Adv Funct Mater. 2016;26:191–9. https://doi.org/10.1002/adfm.201503715.
Sarkar S, Ha YS, Soni N, An GI, Lee W, Kim MH, et al. Immobilization of the gas signaling molecule H2S by radioisotopes: detection, quantification, and in vivo imaging. Angew Chem Int Ed. 2016;55:9365–70. https://doi.org/10.1002/anie.201603813.
Krasnovskaya O, Spector D, Zlobin A, Pavlov K, Gorelkin P, Erofeev A, et al. Metals in imaging of Alzheimer’s disease. Int J Mol Sci. 2020;21:9190. https://doi.org/10.3390/ijms21239190.
McInnes LE, Noor A, Kysenius K, Cullinane C, Roselt P, McLean CA, et al. Potential diagnostic imaging of Alzheimer’s disease with copper-64 complexes that bind to amyloid-beta plaques. Inorg Chem. 2019;58:3382–95. https://doi.org/10.1021/acs.inorgchem.8b03466.
Noor A, Hayne DJ, Lim S, Van Zuylekom JK, Cullinane C, Roselt PD, et al. Copper bis(thiosemicarbazonato)-stilbenyl complexes that bind to amyloid-beta plaques. Inorg Chem. 2020;59:11658–69. https://doi.org/10.1021/acs.inorgchem.0c01520.
Paterson BM, Cullinane C, Crouch PJ, White AR, Barnham KJ, Rose PD, et al. Modification of biodistribution and brain uptake of copper bis(thiosemicarbazonato) complexes by the incorporation of amine and polyamine functional groups. Inorg Chem. 2019;58:4540–52. https://doi.org/10.1021/acs.inorgchem.9b00117.
Paterson BM, Donnelly PS. Copper complexes of bis(thiosemicarbazones): from chemotherapeutics to diagnostic and therapeutic radiopharmaceuticals. Chem Soc Rev. 2011;40:3005–18. https://doi.org/10.1039/c0cs00215a.
Singh NK, Kumbhar AA, Pokharel YR, Yadav PN. Anticancer potency of copper(II) complexes of thiosemicarbazones. J Inorg Biochem. 2020;210: 111134. https://doi.org/10.1016/j.jinorgbio.2020.111134.
Vavere AL, Lewis JS. Cu-ATSM: a radiopharmaceutical for the PET imaging of hypoxia. Dalton Trans. 2007;43:4893–902. https://doi.org/10.1039/b705989b.
Andreozzi EM, Torres JB, Sunassee K, Dunn J, Walker-Samuel S, Szanda I, et al. Studies of copper trafficking in a mouse model of Alzheimer’s disease by positron emission tomography: comparison of 64Cu acetate and 64CuGTSM. Metallomics. 2017;9:1622–33. https://doi.org/10.1039/c7mt00227k.
Hickey JL, Lim S, Hayne DJ, Paterson BM, White JM, Villemagne VL, et al. Diagnostic imaging agents for Alzheimer’s disease: copper radiopharmaceuticals that target abeta plaques. J Am Chem Soc. 2013;135:16120–32. https://doi.org/10.1021/ja4057807.
Takano Y, Shimamoto K, Hanaoka K. Chemical tools for the study of hydrogen sulfide (H2S) and sulfane sulfur and their applications to biological studies. J Clin Biochem Nutr. 2016;58:7–15. https://doi.org/10.3164/jcbn.15-91.
Holland JP, Aigbirhio FI, Betts HM, Bonnitcha PD, Burke P, Christlieb M, et al. Functionalized bis(thiosemicarbazonato) complexes of zinc and copper: synthetic platforms toward site-specific radiopharmaceuticals. Inorg Chem. 2007;46:465–85. https://doi.org/10.1021/ic0615628.
Sarkar S, Bhatt N, Ha YS, Huynh PT, Soni N, Lee W, et al. High in vivo stability of 64Cu-labeled cross-bridged chelators is a crucial factor in improved tumor imaging of RGD peptide conjugates. J Med Chem. 2018;61:385–95. https://doi.org/10.1021/acs.jmedchem.7b01671.
Zhao J, Chen J, Ma S, Liu Q, Huang L, Chen X, et al. Recent developments in multimodality fluorescence imaging probes. Acta Pharm Sin B. 2018;8:320–38. https://doi.org/10.1016/j.apsb.2018.03.010.
Xi L, Jiang H. Image-guided surgery using multimodality strategy and molecular probes. WIREs Nanomed Nanobiotechnol. 2016;8:46–60. https://doi.org/10.1002/wnan.1352.
Velusamy N, Binoy A, Bobba KN, Nedungadi D, Mishra N, Bhuniya S. A bioorthogonal fluorescent probe for mitochondrial hydrogen sulfide: new strategy for cancer cell labeling. Chem Commun. 2017;53:8802–5. https://doi.org/10.1039/c7cc05339h.
Chen H, Gong X, Liu X, Li Z, Zhang J, Yang X-F. A nitroso-based fluorogenic probe for rapid detection of hydrogen sulfide in living cells. Sens Actuators B Chem. 2019;281:542–8. https://doi.org/10.1016/j.snb.2018.10.086.
Magde D, Wong R, Seybold PG. Fluorescence quantum yields and their relation to lifetimes of rhodamine 6G and fluorescein in nine solvents: improved absolute standards for quantum yields. Photochem Photobiol. 2002;75:327–34. https://doi.org/10.1562/0031-8655(2002)075%3c0327:Fqyatr%3e2.0.Co;2.
Paul BD, Snyder SH, Kashfi K. Effects of hydrogen sulfide on mitochondrial function and cellular bioenergetics. Redox Biol. 2021;38:101772. https://doi.org/10.1016/j.redox.2020.101772.
Carter RN, Morton NM. Cysteine and hydrogen sulphide in the regulation of metabolism: insights from genetics and pharmacology. J Pathol. 2016;238:321–32. https://doi.org/10.1002/path.4659.
Sun Q, Collins R, Huang S, Holmberg-Schiavone L, Anand GS, Tan CH, et al. Structural basis for the inhibition mechanism of human cystathionine gamma-lyase, an enzyme responsible for the production of H2S. J Biol Chem. 2009;284:3076–85. https://doi.org/10.1074/jbc.M805459200.
Wu Z, Liang D, Tang X. Visualizing hydrogen sulfide in mitochondria and lysosome of living cells and in tumors of living mice with positively charged fluorescent chemosensors. Anal Chem. 2016;88:9213–8. https://doi.org/10.1021/acs.analchem.6b02459.
Kim JY, Sarkar S, Bobba KN, Huynh PT, Bhise A, Yoo J. Development of dansyl based copper(II) complex to detect hydrogen sulfide in hypoxia. Org Biomol Chem. 2019;17:7088–94. https://doi.org/10.1039/c9ob00948e.
Pajouhesh H, Lenz GR. Medicinal chemical properties of successful central nervous system drugs. NeuroRx. 2005;2:541–53. https://doi.org/10.1602/neurorx.2.4.541.
Yang J, Minkler P, Grove D, Wang R, Willard B, Dweik R, et al. Non-enzymatic hydrogen sulfide production from cysteine in blood is catalyzed by iron and vitamin B6. Commun Biol. 2019;2:194. https://doi.org/10.1038/s42003-019-0431-5.
Akahoshi N, Kamata S, Kubota M, Hishiki T, Nagahata Y, Matsuura T, et al. Neutral aminoaciduria in cystathionine beta-synthase-deficient mice; an animal model of homocystinuria. Am J Physiol Renal Physiol. 2014;306:F1462–76. https://doi.org/10.1152/ajprenal.00623.2013.
Lewis JS, McCarthy DW, McCarthy TJ, Fujibayashi Y, Welch MJ. Evaluation of 64Cu-ATSM in vitro and in vivo in a hypoxic tumor model. J Nucl Med. 1999;40:177–83.
Asimakopoulou A, Panopoulos P, Chasapis CT, Coletta C, Zhou ZM, Cirino G, et al. Selectivity of commonly used pharmacological inhibitors for cystathionine synthase (CBS) and cystathionine lyase (CSE). Brit J Pharmacol. 2013;169:922–32. https://doi.org/10.1111/bph.12171.
Shatalin K, Nuthanakanti A, Kaushik A, Shishov D, Peselis A, Shamovsky I, et al. Inhibitors of bacterial H2S biogenesis targeting antibiotic resistance and tolerance. Science. 2021;372:1169–75. https://doi.org/10.1126/science.abd8377.
Moest RR. Hydrogen sulfide determination by the methylene blue method. Anal Chem. 1975;47:1204–5. https://doi.org/10.1021/ac60357a008.
Savelieff MG, Lee S, Liu Y, Lim MH. Untangling amyloid-beta, tau, and metals in Alzheimer’s disease. ACS Chem Biol. 2013;8:856–65. https://doi.org/10.1021/cb400080f.
Jassam YN, Izzy S, Whalen M, McGavern DB, El Khoury J. Neuroimmunology of traumatic brain injury: Time for a paradigm shift. Neuron. 2017;95:1246–65. https://doi.org/10.1016/j.neuron.2017.07.010.
Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, et al. Parkinson disease Nat Rev Dis Primers. 2017;3:17013. https://doi.org/10.1038/nrdp.2017.13.
Jayaraj RL, Azimullah S, Beiram R, Jalal FY, Rosenberg GA. Neuroinflammation: friend and foe for ischemic stroke. J Neuroinflammation. 2019;16:142. https://doi.org/10.1186/s12974-019-1516-2.
Chun H, Im H, Kang YJ, Kim Y, Shin JH, Won W, et al. Severe reactive astrocytes precipitate pathological hallmarks of Alzheimer’s disease via H2O2- production. Nat Neurosci. 2020;23:1555–66. https://doi.org/10.1038/s41593-020-00735-y.
Hong J, Yoon D, Nam Y, Seo D, Kim JH, Kim MS, et al. Lipopolysaccharide administration for a mouse model of cerebellar ataxia with neuroinflammation. Sci Rep. 2020;10:13337. https://doi.org/10.1038/s41598-020-70390-7.
Jha MK, Jeon S, Jin M, Ock J, Kim JH, Lee WH, et al. The pivotal role played by lipocalin-2 in chronic inflammatory pain. Exp Neurol. 2014;254:41–53. https://doi.org/10.1016/j.expneurol.2014.01.009.
Tao H, Guo J, Ma Y, Zhao Y, Jin T, Gu L, et al. Luminescence imaging of acute liver injury by biodegradable and biocompatible nanoprobes. ACS Nano. 2020;14:11083–99. https://doi.org/10.1021/acsnano.0c00539.
Kumar M, Sandhir R. Hydrogen Sulfide in physiological and pathological mechanisms in brain. CNS Neurol Disord Drug Targets. 2018;17:654–70. https://doi.org/10.2174/1871527317666180605072018.
Eto K, Asada T, Arima K, Makifuchi T, Kimura H. Brain hydrogen sulfide is severely decreased in Alzheimer’s disease. Biochem Biophys Res Commun. 2002;293:1485–8. https://doi.org/10.1016/S0006-291X(02)00422-9.
DiSabato DJ, Quan N, Godbout JP. Neuroinflammation: the devil is in the details. J Neurochem. 2016;139(Suppl 2):136–53. https://doi.org/10.1111/jnc.13607.
Pizza V, Agresta A, D’Acunto CW, Festa M, Capasso A. Neuroinflamm-aging and neurodegenerative diseases: an overview. CNS Neurol Disord Drug Targets. 2011;10:621–34. https://doi.org/10.2174/187152711796235014.
Acknowledgements
The Korea Basic Science Institute is acknowledged for HRMS measurements.
Funding
This work was supported by the R&D program through the National Research Foundation of Korea funded by the Ministry of Science, ICT & Future Planning (Nos. 2020M2D8A3094031, 2019R1A2C2084313, 2017R1A5A2015391, and 2019H1D3A1A01102643). This work was also supported by a grant of the Korea Institute of Radiological and Medical Sciences (KIRAMS), funded by MSIT, Republic of Korea (No. 50461–2022).
Author information
Authors and Affiliations
Contributions
J.Y. conceived and directed the project. J.Y., B.N., W.L., and S.S. designed the experiments. S.S. performed organic synthesis. S.S., A.B., P.T.H., and S.R. performed all radiochemistry works. B.N., W.L., K.L., Y.S.H., J.E.L., and K.W.K. performed imaging and biodistribution studies. B.N., S.H.C., and W.L. performed all of the biological evaluation experiments. H.P., J.Y.K., and K.C.L. produced the Cu-64 isotope. J.-H.K. and K.S. performed neuroinflammation studies. B.N., W.L., S.S., J.-H.K., and J.Y. wrote the manuscript in close collaboration with the other coauthors.
Corresponding author
Ethics declarations
Ethics approval
All animal experiments were performed according to approved animal protocols and guidelines established by the Animal Care Committee of Kyungpook National University (No. KNU 2017–0096, 2019–0009, 2019–0101, 2020–0079).
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Infection and inflammation.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nam, B., Lee, W., Sarkar, S. et al. In vivo detection of hydrogen sulfide in the brain of live mouse: application in neuroinflammation models. Eur J Nucl Med Mol Imaging 49, 4073–4087 (2022). https://doi.org/10.1007/s00259-022-05854-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-022-05854-1