Abstract
Purpose
Bacterial infection and antibiotic resistance are serious threats to human health. This study aimed to develop two novel radiotracers, 18F-NTRP and 18F-NCRP, that possess a specific nitroreductase (NTR) response to image deep-seated bacterial infections using positron emission tomography (PET). This method can distinguish infection from sterile inflammation.
Methods
18F-NTRP and 18F-NCRP were synthesized via a one-step method; all the steps usually involved in tracer radiosynthesis were successfully adapted in the All-In-One automated module. After the physiochemical properties of 18F-NTRP and 18F-NCRP were characterized, their specificity and selectivity for NTR were verified in E. coli and S. aureus. The ex vivo biodistribution of the tracers was evaluated in normal mice. MicroPET-CT imaging was performed in mouse models of bacterial infection and inflammation after the administration of 18F-NTRP or 18F-NCRP.
Results
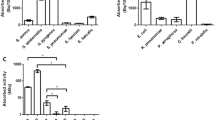
Fully automated radiosynthesis of 18F-NTRP and 18F-NCRP was achieved within 90–110 min with overall decay-uncorrected, isolated radiochemical yields of 21.24 ± 4.25% and 11.3 ± 3.78%, respectively. The molar activities of 18F-NTRP and 18F-NCRP were 320 ± 40 GBq/μmol and 275 ± 33 GBq/µmol, respectively. In addition, 18F-NTRP and 18F-NCRP exhibited high selectivity and specificity for NTR response. PET-CT imaging in bacteria-infected mouse models with 18F-NTRP or 18F-NCRP showed significant radioactivity uptake in either E. coli– or S. aureus–infected muscles. The uptake for E. coli–infected muscles, 2.4 ± 0.2%ID/g with 18F-NTRP and 4.05 ± 0.49%ID/g with 18F-NCRP, was up to three times greater than that for uninfected control muscles. Furthermore, for both 18F-NTRP and 18F-NCRP, the uptake in bacterial infection was 2.6 times higher than that in sterile inflammation, allowing an effective distinction of infection from inflammation.
Conclusion
18F-NTRP and 18F-NCRP are worth further investigation to verify their potential clinical application for distinguishing bacterial infection from sterile inflammation via their specific NTR responsiveness.







Similar content being viewed by others
References
Morens DM, Fauci AS. Emerging infectious diseases: threats to human health and global stability. PLoS Pathog. 2013;9:e1003467.
Carlet J, Collignon P, Goldmann D, et al. Society’s failure to protect a precious resource: antibiotics. Lancet. 2011;378:369–71.
Medina E, Pieper DH. Tackling threats and future problems of multidrug-resistant bacteria. Curr Top Microbiol Immunol. 2016;398:3–33.
van Doorn LJ, Henskens Y, Nouhan N, et al. The efficacy of laboratory diagnosis of helicobacter pylori infections in gastric biopsy specimens is related to bacterial density and vacA, cagA, and iceA genotypes. J Clin Microbiol. 2000;38:13–7.
Li Y, Daryaee F, Yoon GE, et al. Positron emission tomography imaging of Staphylococcus aureus infection using a nitro-prodrug analog of 2-[18F]F-p-aminobenzoic acid. Acs Infect Dis. 2020;6:2249–59.
van Osten M, Hahn M, Crane LM, et al. Targeted imaging of bacterial infections: advances, hurdles and hopes. FEMS Microbiol Rev. 2015;39:892–916.
Gordon O, Ruiz-Bedoya CA, Ordonez AA, Tucker EW, Jain SK, Molecular imaging: a novel tool to visualize pathogenesis of infections in situ. mBio. 2019;10:e00317–19.
James ML, Gambhir SS. A molecular imaging primer: modalities, imaging agents, and applications. Physiol Rev. 2012;92:897–965.
Jain SK. The promise of molecular imaging in the study and treatment of infectious diseases. Mol Imaging Biol. 2017;19:341–7.
Signore A, Glaudemans AW. The molecular imaging approach to image infections and inflammation by nuclear medicine techniques. Ann Nucl Med. 2011;25:681–700.
Polvoy I, Flavell RR, Rosenberg OS, Ohliger MA, Wilson DM. Nuclear imaging of bacterial infection: the state of the art and future directions. J Nucl Med. 2020;61:1708–16.
Mota F, Ordonez AA, Firth G, et al. Radiotracer development for bacterial imaging. J Med Chem. 2020;63:1964–77.
Bunschoten A, Welling MM, Termaat MF, Sathekge M, van Leeuwen FW. Development and prospects of dedicated tracers for the molecular imaging of bacterial infections. Bioconjug Chem. 2013;24:1971–89.
Weinstein EA, Ordonez AA, Demarco VP, et al. Imaging enterobacteriaceae infection in vivo with 18F-fluorodeoxysorbitol positron emission tomography. Sci Transl Med. 2014;6:259ra146.
Yao S, Xing H, Zhu W, et al. Infection imaging with 18F-FDS and first-in-human evaluation. Nucl Med Biol. 2016;43:206–14.
Ning X, Seo W, Lee S, et al. PET imaging of bacterial infections with fluorine-18-labeled maltohexaose. Angew Chem Int Ed Engl. 2014;53:14096–101.
Gowrishankar G, Hardy J, Wardak M, et al. Specific imaging of bacterial infection using 6″-18F-fluoromaltotriose: a second-generation PET tracer targeting the maltodextrin transporter in bacteria. J Nucl Med. 2017;58:1679–84.
Mutch CA, Ordonez AA, Qin H, et al. [11C]Para-aminobenzoic acid: a positron emission tomography tracer targeting bacteria-specific metabolism. ACS Infect Dis. 2018;4:1067–72.
Zhang Z, Ordonez AA, Wang H, et al. Positron emission tomography imaging with 2-[18F]F-p-aminobenzoic acid detects Staphylococcus aureus infections and monitors drug response. ACS Infect Dis. 2018;4:1635–44.
Sellmyer MA, Lee I, Hou C, et al. Bacterial infection imaging with [18F]fluoropropyl-trimethoprim. Proc Natl Acad Sci U S A. 2017;114:8372–7.
Petrik M, Umlaufova E, Raclavsky V, et al. Imaging of pseudomonas aeruginosa infection with Ga-68 labelled pyoverdine for positron emission tomography. Sci Rep. 2018;8:15698.
Zhang XM, Zhang HH, Mcleroth P, et al. [124I]FIAU: human dosimetry and infection imaging in patients with suspected prosthetic joint infection. Nucl Med Biol. 2016;43:273–9.
Parker MFL, Luu JM, Schulte B, et al. Sensing living bacteria in vivo using D-alanine-derived 11C radiotracers. ACS Cent Sci. 2020;6:155–65.
Welling MM, Mongera S, Lupetti A, et al. Radiochemical and biological characteristics of 99mTc-UBI 29–41 for imaging of bacterial infections. Nucl Med Biol. 2002;29:413–22.
Vilche M, Reyes AL, Vasilskis E, et al. Ga-NOTA-UBI-29-41 as a PET tracer for detection of bacterial infection. J Nucl Med. 2016;57:622–7.
Li Z, Li X, Gao X, et al. Nitroreductase detection and hypoxic tumor cell imaging by a designed sensitive and selective fluorescent probe, 7-[(5-Nitrofuran-2-yl)methoxy]-3H-phenoxazin-3-one. Anal Chem. 2013;85:3926–32.
Li Y, Sun Y, Li J, et al. Ultrasensitive near-infrared fluorescence-enhanced probe for in vivo nitroreductase imaging. J Am Chem Soc. 2015;137:6407–16.
Mukherjee A, Rokita SE. Single amino acid switch between a flavin-dependent dehalogenase and nitroreductase. J Am Chem Soc. 2015;137:15342–5.
Wu X, Shi W, Li X, Ma H. Recognition moieties of small molecular fluorescent probes for bioimaging of enzymes. Acc Chem Res. 2019;52:1892–904.
Feng P, Zhang H, Deng Q, et al. Real-time bioluminescence imaging of nitroreductase in mouse model. Anal Chem. 2016;88:5610–4.
Wu LL, Wang Q, Wang Y, et al. Rapid differentiation between bacterial infections and cancer using a near-infrared fluorogenic probe. Chem Sci. 2020;11:3141–5.
Qin W, Xu C, Zhao Y, et al. Recent progress in small molecule fluorescent probes for nitroreductase. Chin Chem Lett. 2018;29:1451–5.
Berlanga M, Montero MT, Hernández-Borrell J, Viñas M. Influence of the cell wall on ciprofloxacin susceptibility in selected wild-type Gram-negative and Gram-positive bacteria. Int J Antimicrob Ag. 2004;23:627–30.
Mirsepasi-Lauridsen HC, Halkjaer SI, Mortensen EM, et al. Extraintestinal pathogenic Escherichia coli are associated with intestinal inflammation in patients with ulcerative colitis. Sci Rep. 2016;6:31152.
Anand S, Mandal S, Singh KS, Patil P, Tomar SK. Synbiotic combination of lactobacillus rhamnosus NCDC 298 and short chain fructooligosaccharides prevents enterotoxigenic Escherichia coli infection. LWT Food Sci Technol. 2018;98:329–34.
Deng X, Rong J, Wang L, et al. Chemistry for positron emission tomography: recent advances in 11C-, 18F-, 13N- and 15O-labeling reactions. Angew Chem Int Ed Engl. 2019;58:2580–605.
Ask K, Décologne N, Asare N, et al. Distribution of nitroreductive activity toward nilutamide in rat. Toxicol Appl Pharmacol. 2004;201:1–9.
Funding
This study was supported by the National Natural Science Foundation of China (21976150), the Major Research Plan of the National Natural Science Foundation of China (91959122), and the Joint Fund of the National Natural Science Foundation of China and the China National Nuclear Corporation for Nuclear Technology Innovation (U1967222).
The experimental procedures, animal use, and care protocols were approved by the Institutional Animal Care and Use Committee of Xiamen University.
Author information
Authors and Affiliations
Contributions
Conceptualization: Xianzhong Zhang, Lumei Huang, Jianyang Fang, Zhide Guo; methodology: Roangqiang Zhuang, Lumei Huang, Shouqiang Hong, and Xilin Zhao; formal analysis and investigation: Lumei Huang, Jianyang Fang, Huanhuan Liu, Haotian Zhu, Lixia Feng; writing (original draft preparation): Lumei Huang, Jianyang Fang; writing (review and editing): Xianzhong Zhang, Zhide Guo, Xilin Zhao; funding acquisition: Xianzhong Zhang, Zhide Guo; supervision: Xianzhong Zhang. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Infection and inflammation
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Huang, L., Fang, J., Hong, S. et al. MicroPET imaging of bacterial infection with nitroreductase-specific responsive 18F-labelled nitrogen mustard analogues. Eur J Nucl Med Mol Imaging 49, 2645–2654 (2022). https://doi.org/10.1007/s00259-022-05710-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-022-05710-2