Abstract
Purpose
To specifically diagnose malignant tumors in DWI using the human telomerase reverse transcriptase (hTERT) promoter–driven AQP1 expression.
Methods
The human telomerase reverse transcriptase (hTERT) promoter–driven AQP1 gene overexpression lentivirus system (hTERT-AQP1) and cytomegalovirus (CMV) promoter–driven AQP1 gene overexpression lentivirus system (CMV-AQP1) were prepared, and transduced into telomerase-positive and -negative cells. The AQP1 expression and DWI signal intensity (SI) change in transduced cells were analyzed. Balb/C nude mice subcutaneous xenograft models derived from lentivirus-transduced telomerase-positive and -negative cells were used to evaluate AQP1 expression and DWI SI change in vivo. We further established another group of subcutaneous xenograft model using pristine telomerase-positive and -negative cells, followed by injecting the lentiviral vectors intratumorally or intravenously, to determine the malignant tumor-targeted imaging of hTERT-AQP1.
Results
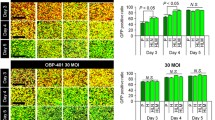
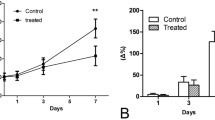
The hTERT-AQP1 and CMV-AQP1 were successfully prepared. After transduction, hTERT-AQP1 could induce the specific overexpression of AQP1 in telomerase-positive cells. Compared with untransduced cells, all CMV-AQP1-pretransduced cells and hTERT-AQP1-pretransduced telomerase-positive cells showed decreased SI and increased apparent diffusion coefficient (ADC) in DWI, while hTERT-AQP1-pretransduced telomerase-negative cells showed no obvious SI and ADC change. Correspondingly, hTERT-AQP1-transduced telomerase-positive tumors and CMV-AQP1-transduced telomerase-positive and -negative tumors showed decreased DWI SI and increased ADC, while hTERT-AQP1-transduced telomerase-negative tumor had no SI and ADC changes. After intratumoral or intravenous injection, CMV-AQP1 could upregulate AQP1 expression and induce DWI SI and ADC alteration in both telomerase-positive and -negative tumors, while hTERT-AQP1 worked in telomerase-positive tumors specifically.
Conclusion
Cancers can be specifically visualized based on the DWI signal alteration which triggered by hTERT-AQP1 lentivirus system that combined AQP1 gene and hTERT promoter.






Similar content being viewed by others
Data availability
Data and materials are available for research purposes from the corresponding authors upon reasonable request.
References
Zhang Y, Zhao J, Yu H, Li P, Liang W, Liu Z, et al. Detection and isolation of free cancer cells from ascites and peritoneal lavages using optically induced electrokinetics (OEK). Sci Adv. 2020;6(32):eaba9628. https://doi.org/10.1126/sciadv.aba9628.
Fontanillas P, Alipanahi B, Furlotte NA, Johnson M, Wilson CH, 23andMe Research Team, et al. Disease risk scores for skin cancers. Nat Commun. 2021;12(1):160. https://doi.org/10.1038/s41467-020-20246-5.
Zhao H, Ke Z, Yang F, Li K, Chen N, Song L, et al. Deep learning enables superior photoacoustic imaging at ultralow laser dosages. Adv Sci. 2021;8(3):2003097. https://doi.org/10.1002/advs.202003097.
Ulaner GA, Sobol NB, O’Donoghue JA, Kirov AS, Riedl CC, Min R, et al. CD38-targeted immuno-PET of multiple myeloma: from xenograft models to first-in-human imaging. Radiology. 2020;295(3):606–15. https://doi.org/10.1148/radiol.2020192621.
Savic LJ, Doemel LA, Schobert IT, Montgomery RR, Joshi N, Walsh JJ, et al. Molecular MRI of the immuno-metabolic interplay in a rabbit liver tumor model: a biomarker for resistance mechanisms in tumor-targeted therapy? Radiology. 2020;296(3):575–83. https://doi.org/10.1148/radiol.2020200373.
Wang R, Yang H, Fu R, Su Y, Lin X, Jin X, et al. Biomimetic upconversion nanoparticles and gold nanoparticles for novel simultaneous dual-modal imaging-guided photothermal therapy of cancer. Cancers. 2020;12(11):3136. https://doi.org/10.3390/cancers12113136.
Chen X, Zhou H, Li X, Duan N, Hu S, Liu Y, et al. Plectin-1 targeted dual-modality nanoparticles for pancreatic cancer imaging. EBioMedicine. 2018;30:129–37. https://doi.org/10.1016/j.ebiom.2018.03.008.
Yu ST, Yang YB, Liang GP, Li C, Chen L, Shi CM, et al. An optimized telomerase-specific lentivirus for optical imaging of tumors. Cancer Res. 2010;70(7):2585–94. https://doi.org/10.1158/0008-5472.CAN-09-3841.
Yang Y, Gong MF, Yang H, Zhang S, Wang GX, Su TS, et al. MR molecular imaging of tumours using ferritin heavy chain reporter gene expression mediated by the hTERT promoter. Eur Radiol. 2016;26(11):4089–97. https://doi.org/10.1007/s00330-016-4259-9.
Wadajkar AS, Dancy JG, Roberts NB, Connolly NP, Strickland DK, Winkles JA, et al. Decreased non-specific adhesivity, receptor targeted (DART) nanoparticles exhibit improved dispersion, cellular uptake, and tumor retention in invasive gliomas. J Control Release. 2017;267:144–53. https://doi.org/10.1016/j.jconrel.2017.09.006.
Gu FX, Karnik R, Wang AZ, Alexis F, Levy-Nissenbaum E, Hong S, et al. Targeted nanoparticles for cancer therapy. Nano Today. 2007;2(3):14–21. https://doi.org/10.1016/S1748-0132(07)70083-X.
Medici S, Peana M, Pelucelli A, Zoroddu MA. An updated overview on metal nanoparticles toxicity. Semin Cancer Biol. 2021;76:17–26. https://doi.org/10.1016/j.semcancer.2021.06.020.
Bondarenko O, Mortimer M, Kahru A, Feliu N, Javed I, Kakinen A, et al. Nanotoxicology and nanomedicine: the Yin and Yang of nano-bio interactions for the new decade. Nano Today. 2021;39:101184. https://doi.org/10.1016/j.nantod.2021.101184.
Youn H, Chung J-K. Reporter gene imaging. Am J Roentgenol. 2013;201(2):W206–14. https://doi.org/10.2214/AJR.13.10555.
Dammes N, Peer D. Monoclonal antibody-based molecular imaging strategies and theranostic opportunities. Theranostics. 2020;10(2):938–55. https://doi.org/10.7150/thno.37443.
Farrar CT, Buhrman JS, Liu G, Kleijn A, Lamfers ML, McMahon MT, et al. Establishing the lysine-rich protein CEST reporter gene as a CEST MR imaging detector for oncolytic virotherapy. Radiology. 2015;275(3):746–54. https://doi.org/10.1148/radiol.14140251.
Pereira SM, Moss D, Williams SR, Murray P, Taylor A. Overexpression of the MRI reporter genes ferritin and transferrin receptor affect iron homeostasis and produce limited contrast in mesenchymal stem cells. Int J Mol Sci. 2015;16(7):15481–96. https://doi.org/10.3390/ijms160715481.
Mukherjee A, Wu D, Davis HC, Shapiro MG. Non-invasive imaging using reporter genes altering cellular water permeability. Nat Commun. 2016;7:13891. https://doi.org/10.1038/ncomms13891.
Tan Y, Zhang H, Wang XC, Qin JB, Wang L. The value of multi ultra high-b-value DWI in grading cerebral astrocytomas and its association with aquaporin-4. Br J Radiol. 2018;91(1086):20170696. https://doi.org/10.1259/bjr.20170696.
Wang Y, Zhang H, Zhang R, Zhao Z, Xu Z, Wang L, et al. Investigation of aquaporins and apparent diffusion coefficient from ultra-high b-values in a rat model of diabetic nephropathy. Eur Radiol Exp. 2017;1(1):13. https://doi.org/10.1186/s41747-017-0016-3.
Yang B, Verkman AS. Water and glycerol permeabilities of aquaporins 1–5 and MIP determined quantitatively by expression of epitope-tagged constructs in Xenopus oocytes. J Biol Chem. 1997;272(26):16140–6. https://doi.org/10.1074/jbc.272.26.16140.
Zhang G, Ma W, Dong H, Shu J, Hou W, Guo Y, et al. Based on histogram analysis: ADCaqp derived from ultra-high b-value DWI could be a non-invasive specific biomarker for rectal cancer prognosis. Sci Rep. 2020;10(1):10158. https://doi.org/10.1038/s41598-020-67263-4.
Mukherjee A, Davis HC, Ramesh P, Lu GJ, Shapiro MG. Biomolecular MRI reporters: evolution of new mechanisms. Prog Nucl Magn Reson Spectrosc. 2017;102–103:32–42. https://doi.org/10.1016/j.pnmrs.2017.05.002.
Le Bihan D. Apparent diffusion coefficient and beyond: what diffusion MR imaging can tell us about tissue structure. Radiology. 2013;268(2):318–22. https://doi.org/10.1148/radiol.13130420.
Hoppe C, Bowles JR, Minchington TG, Sutcliffe C, Upadhyai P, Rattray M, et al. Modulation of the promoter activation rate dictates the transcriptional response to graded BMP signaling levels in the Drosophila embryo. Dev Cell. 2020;54(6):727-741.e727. https://doi.org/10.1016/j.devcel.2020.07.007.
Slusher AL, Kim JJ, Ludlow AT. The role of alternative RNA splicing in the regulation of hTERT, telomerase, and telomeres: implications for cancer therapeutics. Cancers. 2020;12(6):1514. https://doi.org/10.3390/cancers12061514.
Stewart SA, Weinberg RA. Telomerase and human tumorigenesis. Semin Cancer Biol. 2000;10(6):399–406. https://doi.org/10.1006/scbi.2000.0339.
Masutomi K, Hahn WC. Telomerase and tumorigenesis. Cancer Lett. 2003;194(2):163–72. https://doi.org/10.1016/s0304-3835(02)00703-6.
Kyo S, Takakura M, Fujiwara T, Inoue M. Understanding and exploiting hTERT promoter regulation for diagnosis and treatment of human cancers. Cancer Sci. 2008;99(8):1528–38. https://doi.org/10.1111/j.1349-7006.2008.00878.x.
Agarwal N, Rinaldetti S, Cheikh BB, Zhou Q, Hass EP, Jones RT, et al. TRIM28 is a transcriptional activator of the mutant TERT promoter in human bladder cancer. Proc Natl Acad Sci. 2021;118(38):e2102423118. https://doi.org/10.1073/pnas.2102423118.
Bajaj S, Kumar MS, Peters G, Mayur Y. Targeting telomerase for its advent in cancer therapeutics. Med Res Rev. 2020;40(5):1871–919. https://doi.org/10.1002/med.21674.
Pauwels K, Gijsbers R, Toelen J, Schambach A, Willard-Gallo K, Verheust C, et al. State-of-the-art lentiviral vectors for research use: risk assessment and biosafety recommendations. Curr Gene Ther. 2009;9(6):459–74. https://doi.org/10.2174/156652309790031120.
D’Costa J, Mansfield SG, Humeau LM. Lentiviral vectors in clinical trials: current status. Curr Opin Mol Ther. 2009;11(5):554–64.
Milone MC, O’Doherty U. Clinical use of lentiviral vectors. Leukemia. 2018;32(7):1529–41. https://doi.org/10.1038/s41375-018-0106-0.
Gouvarchin Ghaleh HE, Bolandian M, Dorostkar R, Jafari A, Pour MF. Concise review on optimized methods in production and transduction of lentiviral vectors in order to facilitate immunotherapy and gene therapy. Biomed Pharmacother. 2020;128:110276. https://doi.org/10.1016/j.biopha.2020.110276.
Padmanabhan P, Otero J, Ray P, Paulmurugan R, Hoffman AR, Gambhir SS, et al. Visualization of telomerase reverse transcriptase (hTERT) promoter activity using a trimodality fusion reporter construct. J Nucl Med. 2006;47(2):270–7.
Zhong Y, Meng F, Deng C, Zhong Z. Ligand-directed active tumor-targeting polymeric nanoparticles for cancer chemotherapy. Biomacromol. 2014;15(6):1955–69. https://doi.org/10.1021/bm5003009.
Manoharan D, Das CJ, Aggarwal A, Gupta AK. Diffusion weighted imaging in gynecological malignancies-present and future. World J Radiol. 2016;8(3):288–97. https://doi.org/10.4329/wjr.v8.i3.288.
Heidemeier A, Thurner A, Metz C, Pabst T, Heidemeier H, Rasche L, et al. Whole-body MRI with an ultrahigh b-value of 2000 s/mm2 improves the specificity of diffusion-weighted imaging in patients with plasma cell dyscrasias. Acad Radiol. 2022;29(1):e1–8. https://doi.org/10.1016/j.acra.2020.09.016.
Gatidis S, Schmidt H, Martirosian P, Nikolaou K, Schwenzer NF. Apparent diffusion coefficient-dependent voxelwise computed diffusion-weighted imaging: an approach for improving SNR and reducing T2 shine-through effects. J Magn Reson Imaging. 2016;43(4):824–32. https://doi.org/10.1002/jmri.25044.
Funding
This research was supported by Natural Science Foundation of Chongqing, China (cstc2018jcyjAX0321 and cstc2021jcyj-msxmX1093), Natural Science Foundation of Army Medical University (No. 2019R059 and 2019R020), and Talents project of ChongQing, China (Dong Zhang).
Author information
Authors and Affiliations
Contributions
LZ, MG, and DZ designed the experiments; LZ and MG prepared the lentivirus; LZ and MG analyzed AQP1 expression; LZ, SL, CC, and SZ conducted the MRI experiments, LZ and ZX established the tumor models; LZ, MG, YL, SX, XK, and TS performed statistical and MRI analysis; LZ, MG, SL, CZ, and DZ interpreted data and wrote, reviewed, revised manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval
All experiments involving animals were performed following the National Institutes of Health guidelines on the use of animals in research and were approved by the Laboratory Animal Welfare and Ethics Committee of the Army Medical University, Chongqing, China.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Theragnostic
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, L., Gong, M., Lei, S. et al. Targeting visualization of malignant tumor based on the alteration of DWI signal generated by hTERT promoter–driven AQP1 overexpression. Eur J Nucl Med Mol Imaging 49, 2310–2322 (2022). https://doi.org/10.1007/s00259-022-05684-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-022-05684-1