Abstract
Purpose
The aim of this study was to determine a better criterion for end-of-treatment PET (EoT-PET) assessment and prognostic evaluation of patients with diffuse large B cell lymphoma (DLBCL).
Method
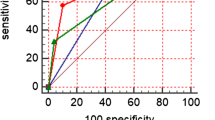
EoT-PET scans were assessed using the visual Deauville 5-point scale (5PS) and LLR, the maximum standard uptake value ratio between the lesion and the liver. The cutoff value of LLR was obtained by receiver operator characteristic curve analysis. Patient outcomes were compared using Kaplan–Meier survival analysis. Prognostic indexes of different criteria were compared. Multivariate Cox regression analysis was performed to evaluate the prognostic factors.
Results
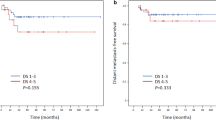
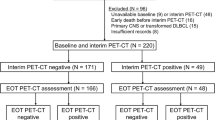
Four hundred forty-nine newly diagnosed DLBCL patients who received rituximab-based immunochemotherapy were included, and the median follow-up duration was 41.4 months. Patients with Deauville score (DS) 4 displayed significantly longer PFS and OS compared with patients with DS 5 (both p < 0.001), and they had significantly shorter PFS (p < 0.01) but similar OS (p = 0.057) compared with patients with DS 1–3. The differences in PFS and OS between groups were all significant whether positive EoT-PET was defined as DS 4–5 or DS 5 (all p < 0.001). The optimal cutoff of LLR was 1.83, and both PFS and OS were significantly different between EoT-PET-positive and EoT-PET-negative patients as defined by the cutoff (both p < 0.001). LLR-based criterion displayed higher specificity, positive predictive value, and accuracy than 5PS-based criterion in the prediction of disease progression and death events. In the multivariate analysis, positive EoT-PET (as defined by LLR) was related to unfavorable PFS and OS (both p < 0.001). Additional treatment was not correlated with outcomes of EoT-PET-negative patients either defined by LLR or 5PS or EoT-PET-positive patients classified by 5PS, but it was the only beneficial factor for OS (p < 0.05) in EoT-PET-positive patients with LLR ≥ 1.83.
Conclusion
The optimal cutoff of LLR may be superior to Deauville criteria in identifying low-risk DLBCL patients with negative EoT-PET after the first-line immunochemotherapy and sparing them the cost and toxicity of additional treatment.




Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Al-Hamadani M, Habermann TM, Cerhan JR, Macon WR, Maurer MJ, Go RS. Non-Hodgkin lymphoma subtype distribution, geodemographic patterns, and survival in the US: a longitudinal analysis of the National Cancer Data Base from 1998 to 2011. Am J Hematol. 2015;90:790–5. https://doi.org/10.1002/ajh.24086.
Perry AM, Diebold J, Nathwani BN, Maclennan KA, Müller-Hermelink HK, Bast M, et al. Non-Hodgkin lymphoma in the developing world: review of 4539 cases from the International Non-Hodgkin Lymphoma Classification Project. Haematologica. 2016;101:1244–50. https://doi.org/10.3324/haematol.2016.148809.
Weiler-Sagie M, Bushelev O, Epelbaum R, Dann EJ, Haim N, Avivi I, et al. 18F-FDG avidity in lymphoma readdressed: a study of 766 patients. J Nuc Med. 2010;51:25–30. https://doi.org/10.2967/jnumed.109.067892.
Zelenetz AD, Gordon LI, Wierda WG, Abramson JS, Advani RH, Andreadis CB, et al. Diffuse large B-cell lymphoma version 1.2016. J Natl Compr Canc Netw. 2016;14:196–231. https://doi.org/10.6004/jnccn.2016.0023.
Zelenetz AD, Gordon LI, Abramson JS, Advani RH, Bartlett NL, Caimi PF, et al. NCCN guidelines insights: B-cell lymphomas, Version 3.2019. J Natl Compr Canc Netw. 2019;17:650–61. https://doi.org/10.6004/jnccn.2019.0029.
Tilly H, Gomes da Silva M, Vitolo U, Jack A, Meignan M, Lopez-Guillermo A, et al. Diffuse large B-cell lymphoma (DLBCL): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26 Suppl 5:v116–25. https://doi.org/10.1093/annonc/mdv304.
Barrington SF, Mikhaeel NG, Kostakoglu L, Meignan M, Hutchings M, Müeller SP, et al. Role of imaging in the staging and response assessment of lymphoma: consensus of the international conference on malignant lymphomas imaging working group. J Clin Oncol. 2014;32:3048–58. https://doi.org/10.1200/JCO.2013.53.5229.
Kostakoglu L, Nowakowski GS. End-of-treatment PET/computed tomography response in diffuse large B-cell lymphoma. PET clinics. 2019;14:307–15. https://doi.org/10.1016/j.cpet.2019.03.001.
Kobe C, Dietlein M, Hellwig D. PET/CT for lymphoma post-therapy response assessment in Hodgkin lymphoma and diffuse large B-cell lymphoma. Semin Nucl Med. 2018;48:28–36. https://doi.org/10.1053/j.semnuclmed.2017.09.003.
Melani C, Advani R, Roschewski M, Walters KM, Chen CC, Baratto L, et al. End-of-treatment and serial PET imaging in primary mediastinal B-cell lymphoma following dose-adjusted EPOCH-R: a paradigm shift in clinical decision making. Haematologica. 2018;103:1337–44. https://doi.org/10.3324/haematol.2018.192492.
Freeman CL, Savage KJ, Villa DR, Scott DW, Srour L, Gerrie AS, et al. Long-term results of PET-guided radiation in patients with advanced-stage diffuse large B-cell lymphoma treated with R-CHOP. Blood. 2021;137:929–38. https://doi.org/10.1182/blood.2020005846.
Adams HJA, Kwee TC. Systematic review on the value of end-of-treatment FDG-PET in improving overall survival of lymphoma patients. Ann Hematol. 2020;99:1–5. https://doi.org/10.1007/s00277-019-03881-x.
Adams HJA, Nievelstein RAJ, Kwee TC. Prognostic value of complete remission status at end-of-treatment FDG-PET in R-CHOP-treated diffuse large B-cell lymphoma: systematic review and meta-analysis. Br J Haematol. 2015;170:185–91. https://doi.org/10.1111/bjh.13420.
Kostakoglu L, Martelli M, Sehn LH, Belada D, Carella AM, Chua N, et al. End-of-treatment PET/CT predicts PFS and OS in DLBCL after first-line treatment: results from GOYA. Blood Adv. 2021;5:1283–90. https://doi.org/10.1182/bloodadvances.2020002690.
Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano classification. J Clin Oncol. 2014;32:3059–67. https://doi.org/10.1200/JCO.2013.54.8800.
Meignan M, Gallamini A, Haioun C. Report on the First International Workshop on interim-PET scan in lymphoma. Leuk Lymphoma. 2009;50:1257–60. https://doi.org/10.1080/10428190903040048.
Armitage JO, Gascoyne RD, Lunning MA, Cavalli F. Non-Hodgkin lymphoma. Lancet. 2017;390:298–310. https://doi.org/10.1016/s0140-6736(16)32407-2.
Burggraaff CN, Cornelisse AC, Hoekstra OS, Lugtenburg PJ, De Keizer B, Arens AIJ, et al. Interobserver agreement of interim and end-of-treatment18F-FDG PET/CT in diffuse large B-cell lymphoma: Impact on clinical practice and trials. J Nuc Med. 2018;59:1831–6. https://doi.org/10.2967/jnumed.118.210807.
Zhang Y, Fan Y, Ying Z, Song Y, Zhu J, Yang Z, et al. Can the LLR-based interpretation improve prognostic accuracy of interim and posttreatment 18F-FDG PET/CT in patients with diffuse large B-cell lymphoma? Leuk Lymphoma. 2018;59:660–9. https://doi.org/10.1080/10428194.2017.1357171.
Toledano MN, Vera P, Tilly H, Jardin F, Becker S. Comparison of therapeutic evaluation criteria in FDG-PET/CT in patients with diffuse large-cell B-cell lymphoma: prognostic impact of tumor/liver ratio. PLoS ONE. 2019;14. https://doi.org/10.1371/journal.pone.0211649.
Annunziata S, Pelliccioni A, Hohaus S, Maiolo E, Cuccaro A, Giordano A. The prognostic role of end-of-treatment FDG-PET/CT in diffuse large B cell lymphoma: a pilot study application of neural networks to predict time-to-event. Ann Nucl Med. 2021;35:102–10. https://doi.org/10.1007/s12149-020-01542-y.
Juweid ME, Stroobants S, Hoekstra OS, Mottaghy FM, Dietlein M, Guermazi A, et al. Use of positron emission tomography for response assessment of lymphoma: consensus of the imaging subcommittee of international harmonization project in lymphoma. J Clin Oncol. 2007;25:571–8. https://doi.org/10.1200/JCO.2006.08.2305.
Project IN-HsLPF. A predictive model for aggressive non-Hodgkin’s lymphoma. N Engl J Med. 1993;329:987–94. https://doi.org/10.1056/nejm199309303291402.
Lee JW, Oh D, Eom KY, Kim JH, Kim WC, Chung MJ, et al. The prognostic value of PET/CT evaluation with Deauville score on the recurrence and survival in diffuse large B-cell lymphoma: a multi-institutional study of KROG 17–02. Clin Exp Metastasis. 2020;37:125–31. https://doi.org/10.1007/s10585-019-09992-z.
Del Puig Cózar-Santiago M, García-Garzón JR, Moragas-Freixa M, Soler-Peter M, Bassa Massanas P, Sánchez-Delgado M, et al. Optimisation of metabolic criteria in the prognostic assessment in patients with lymphoma. A multicentre study. Rev Esp Med Nucl Imagen Mol. 2017;36:304–11. https://doi.org/10.1016/j.remn.2017.03.003.
Schöder H, Polley MC, Knopp MV, Hall N, Kostakoglu L, Zhang J, et al. Prognostic value of interim FDG-PET in diffuse large cell lymphoma: results from the CALGB 50303 Clinical Trial. Blood. 2020;135:2224–34. https://doi.org/10.1182/blood.2019003277.
Meignan M, Gallamini A, Itti E, Barrington S, Haioun C, Polliack A. Report on the Third International Workshop on Interim Positron Emission Tomography in Lymphoma held in Menton, France, 26–27 September 2011 and Menton 2011 consensus. Leuk Lymphoma. 2012;53:1876–81. https://doi.org/10.3109/10428194.2012.677535.
Specht L. Does radiation have a role in advanced stage Hodgkin’s or non-Hodgkin lymphoma? Curr Treat Options Oncol. 2016;17:4. https://doi.org/10.1007/s11864-015-0377-x.
Ballonoff A, Rusthoven KE, Schwer A, McCammon R, Kavanagh B, Bassetti M, et al. Outcomes and effect of radiotherapy in patients with stage I or II diffuse large B-cell lymphoma: a surveillance, epidemiology, and end results analysis. Int J Radiat Oncol Biol Phys. 2008;72:1465–71. https://doi.org/10.1016/j.ijrobp.2008.02.068.
Vargo JA, Gill BS, Balasubramani GK, Beriwal S. Treatment selection and survival outcomes in early-stage diffuse large B-cell lymphoma: do we still need consolidative radiotherapy? J Clin Oncol. 2015;33:3710–7. https://doi.org/10.1200/jco.2015.61.7654.
Chung MJ, Cho WK, Oh D, Eom KY, Kim JH, Kim WC, et al. A multi-institutional and case-matched control study on treatment outcomes of consolidative radiotherapy after a full course of R-CHOP compared with R-CHOP alone in stage I-II diffuse large B-cell lymphoma (KROG 17–02). J Radiat Res. 2019;60:677–84. https://doi.org/10.1093/jrr/rrz043.
Tomita N, Kodama F, Motomura S, Itoh S, Ohshima R, Hyo R, et al. Adjuvant radiotherapy to an initial bulky mass in diffuse large B-cell lymphoma: lack of survival benefit. Int J Lab Hematol. 2008;30:53–7. https://doi.org/10.1111/j.1751-553X.2007.00900.x.
Shi Y, Han Y, Yang J, Liu P, He X, Zhang C, et al. Clinical features and outcomes of diffuse large B-cell lymphoma based on nodal or extranodal primary sites of origin: analysis of 1,085 WHO classified cases in a single institution in China. Chin J Cancer Res. 2019;31:152–61. https://doi.org/10.21147/j.issn.1000-9604.2019.01.10.
Li X, Xie X, Zhang L, Li X, Li L, Wang X, et al. Research on the midterm efficacy and prognosis of patients with diffuse large B-cell lymphoma by different evaluation methods in interim PET/CT. Eur J Radiol. 2020;133: 109301. https://doi.org/10.1016/j.ejrad.2020.109301.
Funding
This work was supported by grants from the Medical Science and Technology Foundation of Guangdong Province (A2018077).
Author information
Authors and Affiliations
Contributions
Conceptualization, Xu Zhang, Ying-Ying Hu, and Ying-He Li; methodology, Ying-He Li, Yu-Mo Zhao, Mei-Ting Chen, and Zi-Zheng Xiao; formal analysis and investigation, Ying-He Li, Zi-Zheng Xiao, Yong-Luo Jiang, Mei-Ting Chen, Si Tang, Yu-Mo Zhao, Ying-Ying Hu, and Xu Zhang; writing (original draft preparation), Ying-He Li; writing (review and editing), Ying-He Li, Yu-Mo Zhao, and Yong-Luo Jiang; resources, Wei Fan; supervision, Xu Zhang, Ying-Ying Hu, and Wei Fan. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Ethical approval was also obtained from the Ethics Committee of Sun Yat-Sen University Cancer Center (approval number: B2021-189–01).
Consent to participate
This retrospective study was performed with the Ethics Committee approval and a waiver of the requirement for patients’ informed consent.
Consent for publication
Additional informed consent was obtained from all legal guardians for whom identifying information is included in this article.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Hematology
Ying-He Li, Yu-Mo Zhao and Yong-Luo Jiang contributed equally to this work.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, YH., Zhao, YM., Jiang, YL. et al. The prognostic value of end-of-treatment FDG-PET/CT in diffuse large B cell lymphoma: comparison of visual Deauville criteria and a lesion-to-liver SUVmax ratio-based evaluation system. Eur J Nucl Med Mol Imaging 49, 1311–1321 (2022). https://doi.org/10.1007/s00259-021-05581-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-021-05581-z