Abstract
Purpose
FDG-PET is an established supportive biomarker in dementia with Lewy bodies (DLB), but its diagnostic accuracy is unknown at the mild cognitive impairment (MCI-LB) stage when the typical metabolic pattern may be difficultly recognized at the individual level. Semiquantitative analysis of scans could enhance accuracy especially in less skilled readers, but its added role with respect to visual assessment in MCI-LB is still unknown.
Methods
We assessed the diagnostic accuracy of visual assessment of FDG-PET by six expert readers, blind to diagnosis, in discriminating two matched groups of patients (40 with prodromal AD (MCI-AD) and 39 with MCI-LB), both confirmed by in vivo biomarkers. Readers were provided in a stepwise fashion with (i) maps obtained by the univariate single-subject voxel-based analysis (VBA) with respect to a control group of 40 age- and sex-matched healthy subjects, and (ii) individual odds ratio (OR) plots obtained by the volumetric regions of interest (VROI) semiquantitative analysis of the two main hypometabolic clusters deriving from the comparison of MCI-AD and MCI-LB groups in the two directions, respectively.
Results
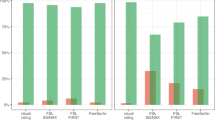
Mean diagnostic accuracy of visual assessment was 76.8 ± 5.0% and did not significantly benefit from adding the univariate VBA map reading (77.4 ± 8.3%) whereas VROI-derived OR plot reading significantly increased both accuracy (89.7 ± 2.3%) and inter-rater reliability (ICC 0.97 [0.96–0.98]), regardless of the readers’ expertise.
Conclusion
Conventional visual reading of FDG-PET is moderately accurate in distinguishing between MCI-LB and MCI-AD, and is not significantly improved by univariate single-subject VBA but by a VROI analysis built on macro-regions, allowing for high accuracy independent of reader skills.





Similar content being viewed by others
References
Boccardi M, Nicolosi V, Festari C, et al. Italian consensus recommendations for a biomarker-based aetiological diagnosis in mild cognitive impairment patients. Eur J Neurol. 2020;27:475–83.
Nobili F, Arbizu J, Bouwman F, et al. European Association of Nuclear Medicine and European Academy of Neurology recommendations for the use of brain 18 F-fluorodeoxyglucose positron emission tomography in neurodegenerative cognitive impairment and dementia: Delphi consensus. Eur J Neurol. 2018;25:1201–17.
Chételat G, Arbizu J, Barthel H, et al. Amyloid-PET and 18F-FDG-PET in the diagnostic investigation of Alzheimer’s disease and other dementias. Lancet Neurol. 2020;19:951–62.
Minoshima S, Foster NL, Sima AAF, Frey KA, Albin RL, Kuhl DE. Alzheimer’s disease versus dementia with Lewy bodies: cerebral metabolic distinction with autopsy confirmation. Ann Neurol. 2001;50:358–65.
O’Brien JT, Firbank MJ, Davison C, et al. 18F-FDG PET and perfusion SPECT in the diagnosis of Alzheimer and Lewy body dementias. J Nucl Med. 2014;55:1959–65.
Kono AK, Ishii K, Sofue K, Miyamoto N, Sakamoto S, Mori E. Fully automatic differential diagnosis system for dementia with Lewy bodies and Alzheimer’s disease using FDG-PET and 3D-SSP. Eur J Nucl Med Mol Imaging. 2007;34:1490–7.
Seok ML, Katsifis A, Villemagne VL, et al. The 18F-FDG PET cingulate island sign and comparison to 123I-β-CIT SPECT for diagnosis of dementia with Lewy bodies. J Nucl Med. 2009;50:1638–45.
Perani D, Della Rosa PA, Cerami C, et al. Validation of an optimized SPM procedure for FDG-PET in dementia diagnosis in a clinical setting. NeuroImage Clin. 2014;6:445–54.
Firbank MJ, Lloyd J, Williams D, et al. An evidence-based algorithm for the utility of FDG-PET for diagnosing Alzheimer’s disease according to presence of medial temporal lobe atrophy. Br J Psychiatry. 2016;208:491–6.
McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies. Neurology. 2017;89:88–100.
Morbelli S, Chincarini A, Brendel M, et al. Metabolic patterns across core features in dementia with Lewy bodies. Ann Neurol. 2019;85:715–25.
Kantarci K, Boeve BF, Przybelski SA, et al. FDG PET metabolic signatures distinguishing prodromal DLB and prodromal AD. NeuroImage Clin. 2021;31:102754.
Bauckneht M, Chincarini A, Brendel M, et al. Associations among education, age, and the dementia with Lewy bodies (DLB) metabolic pattern: a European-DLB consortium project. Alzheimer’s Dement. 2021.
McKeith IG, Ferman TJ, Thomas AJ, et al. Research criteria for the diagnosis of prodromal dementia with Lewy bodies. Neurology. 2020;94:743–55.
Rizzo G, Arcuti S, Copetti M, et al. Accuracy of clinical diagnosis of dementia with Lewy bodies: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2018;89:358–66.
Clerici F, Del Sole A, Chiti A, et al. Differences in hippocampal metabolism between amnestic and non-amnestic MCI subjects: automated FDG-PET image analysis. Q J Nucl Med Mol Imaging. 2009;53:646–57.
Cerami C, Della Rosa PA, Magnani G, et al. Brain metabolic maps in mild cognitive impairment predict heterogeneity of progression to dementia. NeuroImage Clin. 2015;7:187–94.
Arbizu J, Festari C, Altomare D, et al. Clinical utility of FDG-PET for the clinical diagnosis in MCI. Eur J Nucl Med Mol Imaging. 2018;45:1497–508.
Morbelli S, Brugnolo A, Bossert I, et al. Visual versus semi-quantitative analysis of 18F-FDG-PET in amnestic MCI: an European Alzheimer’s Disease Consortium (EADC) Project. J Alzheimer’s Dis. 2015;44:815–26.
Nobili F, Festari C, Altomare D, et al. Automated assessment of FDG-PET for differential diagnosis in patients with neurodegenerative disorders. Eur J Nucl Med Mol Imaging. 2018;45:1557–66.
Whitwell JL, Graff-Radford J, Singh TD, et al. 18 F-FDG PET in posterior cortical atrophy and dementia with Lewy bodies. J Nucl Med. 2017;58:632–8.
Caminiti SP, Sala A, Iaccarino L, et al. Brain glucose metabolism in Lewy body dementia: implications for diagnostic criteria. Alzheimer’s Res Ther. 2019;11:1–14.
Petersen RC, Lopez O, Armstrong MJ, et al. Practice guideline update summary: mild cognitive impairment report of the guideline development, dissemination, and implementation. Neurology. 2018;90:126–35.
Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011;7:270–9.
Wahlund LO, Barkhof F, Fazekas F, et al. A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke. 2001;32:1318–22.
Varrone A, Asenbaum S, Vander Borght T, et al. EANM procedure guidelines for PET brain imaging using [18F]FDG, version 2. Eur J Nucl Med Mol Imaging. 2009;36:2103–10.
Massa F, Grisanti S, Brugnolo A, et al. The role of anterior prefrontal cortex in prospective memory: an exploratory FDG-PET study in early Alzheimer{’}s disease. Neurobiol Aging. 2020.
Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp. 2009;30:2907–26.
Lancaster JL, Woldorff MG, Parsons LM, et al. Automated Talairach atlas labels for functional brain mapping. Vol 10.; 2000.
McKeith I, O’Brien J, Walker Z, et al. Sensitivity and specificity of dopamine transporter imaging with 123I-FP-CIT SPECT in dementia with Lewy bodies: a phase III, multicentre study. Lancet Neurol. 2007;6:305–13.
Thomas AJ, Attems J, Colloby SJ, et al. Autopsy validation of 123 I-FP-CIT dopaminergic neuroimaging for the diagnosis of DLB. Neurology. 2017;88:276–83.
Thomas AJ, Donaghy P, Roberts G, et al. Diagnostic accuracy of dopaminergic imaging in prodromal dementia with Lewy bodies. Psychol Med. 2019;49:396–402.
Perra D, Bongianni M, Novi G, et al. Alpha-synuclein seeds in olfactory mucosa and cerebrospinal fluid of patients with dementia with Lewy bodies. Brain Commun. 2021;3.
Pagani M, De Carli F, Morbelli S, et al. Volume of interest-based [18F]fluorodeoxyglucose PET discriminates MCI converting to Alzheimer’s disease from healthy controls. A European Alzheimer’s Disease Consortium (EADC) study. NeuroImage Clin. 2015;7:34–42.
Brugnolo A, De Carli F, Pagani M, et al. Head-to-head comparison among semi-quantification tools of brain FDG-PET to aid the diagnosis of prodromal Alzheimer’s disease. J Alzheimer’s Dis. 2019;68:383–94.
Ishii K, Imamura T, Sasaki M, et al. Regional cerebral glucose metabolism in dementia with lewy bodies and Alzheimer’s disease. Neurology. 1998;51:125–30.
Higuchi M, Tashiro M, Arai H, et al. Glucose hypometabolism and neuropathological correlates in brains of dementia with Lewy bodies. Exp Neurol. 2000;162:247–56.
Ishii K, Soma T, Kono AK, et al. Comparison of regional brain volume and glucose metabolism between patients with mild dementia with Lewy bodies and those with mild Alzheimer’s disease. J Nucl Med. 2007;48:704–11.
Bauckneht M, Chincarini A, Piva R, et al. Metabolic correlates of reserve and resilience in MCI due to Alzheimer’s Disease (AD) Rik Ossenkoppele. Alzheimer’s Res Ther. 2018;10.
Gjerum L, Frederiksen KS, Henriksen OM, et al. A visual rating scale for cingulate island sign on 18F-FDG-PET to differentiate dementia with Lewy bodies and Alzheimer’s disease. J Neurol Sci. 2020;410:116645.
Gomperts SN, Locascio JJ, Marquie M, et al. Brain amyloid and cognition in Lewy body diseases. Mov Disord. 2012;27:965–73.
Donaghy PC, Firbank MJ, Thomas AJ, et al. Amyloid imaging and longitudinal clinical progression in dementia with Lewy bodies. Am J Geriatr Psychiatry. 2020;28:573–7.
Lemstra AW, De Beer MH, Teunissen CE, et al. Concomitant AD pathology affects clinical manifestation and survival in dementia with Lewy bodies. J Neurol Neurosurg Psychiatry. 2017;88:113–8.
Kantarci K, Lowe VJ, Chen Q, et al. β-Amyloid PET and neuropathology in dementia with Lewy bodies. Neurology. 2020;94:e282-e291.
Etminani K, Soliman A, Davidsson A, et al. A 3D deep learning model to predict the diagnosis of dementia with Lewy bodies, Alzheimer’s disease, and mild cognitive impairment using brain 18F-FDG PET. Eur J Nucl Med Mol Imaging. 2021.
Twohig, D, Nielsen HM (2019) α-synuclein in the pathophysiology of Alzheimer’s disease. Molecular Neurodegeneration 14(1) https://doi.org/10.1186/s13024-019-0320-x
Acknowledgements
This work was developed within the framework of the DINOGMI Department of Excellence of MIUR 2018-2022 (legge 232 del 2016).
Funding
This work received fund support by the Italian Ministry of Health (Fondi per la Ricerca Corrente, 2020).
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and design of the study and to data acquisition and analysis. The first draft of the manuscript was written by FM and AC and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
All procedures were performed in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. An institutional review board statement was not necessary because we merely used data collected during clinical routine and no other supplementary examinations were performed.
Informed consent and consent for publication
Informed consent was obtained from all individual participants included in the study. All subjects gave their consent to publish their anonymized data.
Conflict of interest
Silvia Morbelli has received speaker Honoraria from G.E. Healthcare; Dario Arnaldi received fees from Fidia for lectures and board participation; Matteo Pardini receives research support from Novartis and Nutricia; received fees from Novartis, Merck, and Biogen; and is partly supported by a University of Genoa “curiosity-driven” grant; Flavio Nobili has received fees for participating in boards from Roche, and speaker Honoraria from Bial e G.E. Healthcare. The other authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Neurology
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Massa, F., Chincarini, A., Bauckneht, M. et al. Added value of semiquantitative analysis of brain FDG-PET for the differentiation between MCI-Lewy bodies and MCI due to Alzheimer’s disease. Eur J Nucl Med Mol Imaging 49, 1263–1274 (2022). https://doi.org/10.1007/s00259-021-05568-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-021-05568-w