Abstract
Purpose
To evaluate the role of positron emission tomography/computed tomography (PET/CT) in predicting pathologic complete response (pCR) and identify relevant prognostic factors from clinico-imaging-pathologic features of locally advanced esophageal squamous cell carcinoma (eSCC) patients undergoing trimodality therapy.
Methods
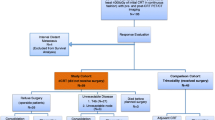
We evaluated 275 patients with eSCCs of T3-T4aN0M0 and T1-T4aN1-N3M0 who received trimodality therapy. We correlated volume-based PET/CT parameters before and after concurrent chemoradiation therapy with pCR after surgery, clinico-imaging-pathologic features, and patient survival.
Results
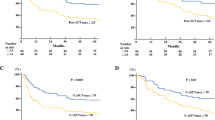
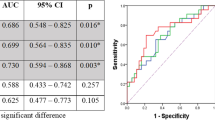
pCR occurred in 75 (27.3%) of 275 patients, of whom 61 (80.9%) showed 5-year survival. Pre-total lesion glycolysis (pre-TLG, OR = 0.318, 95% CI 0.169 to 0.600), post-metabolic tumor volume (post-MTV, OR = 0.572, 95% CI 0.327 to 0.999), and % decrease of average standardized uptake value (% SUVavg decrease, OR = 2.976, 95% CI = 1.608 to 5.507) were significant predictors for pCR. Among them, best predictor for pCR was pre-TLG with best cutoff value of 205.67 and with AUC value of 0.591.
Performance status (HR = 5.171, 95% CI 1.737 to 15.397), pathologic tumor size (HR = 1.645, 95% CI 1.351 to 2.002), pathologic N status (N1, HR = 1.572, 95% CI 1.010 to 2.446; N2, HR = 3.088, 95% CI 1.845 to 5.166), and post-metabolic tumor volume (HR = 1.506, 95% CI 1.033 to 2.195) were significant predictors of overall survival.
Conclusion
Pre-TLG, post-MTV, and % SUVavg decrease are predictive of pCR. Additionally, several clinico-imaging-pathologic factors are significant survival predictors in locally advanced eSCC patients undergoing trimodality therapy.




Similar content being viewed by others
Data availability
Not applicable.
Code availability
Not applicable.
Abbreviations
- CCRT:
-
Concurrent chemoradiation therapy
- CI :
-
Confidence interval
- DFS :
-
Disease-free survival
- EUS:
-
Endoscopic ultrasonography
- MTV :
-
Metabolic tumor volume
- eSCC :
-
Esophageal squamous cell carcinoma
- LVI :
-
Lymphovascular invasion
- OR :
-
Odds ratio
- OS :
-
Overall survival
- PNI :
-
Perineural invasion
- pCR :
-
Pathologic complete response
- RNL:
-
Recurrent laryngeal nerve
- SD:
-
Standard deviation
- SUV:
-
Standardized uptake value
- SUVavg :
-
Average standardized uptake value
- SUVmax:
-
Maximum standardized uptake value
- TLG:
-
Total lesion glycolysis
References
Jeong DY, Kim MY, Lee KS, et al. Surgically resected T1- and T2-stage esophageal squamous cell carcinoma: T and N staging performance of EUS and PET/CT. Cancer Med. 2018;7(8):3561–70. https://doi.org/10.1002/cam4.1617.
Jeong DY, Lee KS, Choi JY, et al. Surgically resected esophageal squamous cell carcinoma: patient survival and clinicopathological prognostic factors. Sci Rep. 2020;10(1):5077. https://doi.org/10.1038/s41598-020-62028-5.
Huang YC, Lu HI, Huang SC, et al. FDG PET using SUVmax for preoperative T-staging of esophageal squamous cell carcinoma with and without neoadjuvant chemoradiotherapy. BMC Med Imaging. 2017;17(1):1. https://doi.org/10.1186/s12880-016-0171-7.
Chuang HH, Macapinlac HA. The evolving role of PET-CT in the management of esophageal cancer. Q J Nucl Med Mol Imaging. 2009;53(2):201–9.
Gebski V, Burmeister B, Smithers BM, et al. Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: a meta-analysis. Lancet Oncol. 2007;8(3):226–34. https://doi.org/10.1016/S1470-2045(07)70039-6.
Tepper J, Krasna MJ, Niedzwiecki D, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol. 2008;26(7):1086–92. https://doi.org/10.1200/JCO.2007.12.9593.
Bollschweiler E, Metzger R, Drebber U, et al. Histological type of esophageal cancer might affect response to neo-adjuvant radiochemotherapy and subsequent prognosis. Ann Oncol. 2009;20(2):231–8. https://doi.org/10.1093/annonc/mdn622.
Connors RC, Reuben BC, Neumayer LA, Bull DA. Comparing outcomes after transthoracic and transhiatal esophagectomy: a 5-year prospective cohort of 17,395 patients. J Am Coll Surg. 2007;205(6):735–40. https://doi.org/10.1016/j.jamcollsurg.2007.07.001.
Chang AC, Ji H, Birkmeyer NJ, Orringer MB, Birkmeyer JD. Outcomes after transhiatal and transthoracic esophagectomy for cancer. Ann Thorac Surg. 2008;85(2):424–9. https://doi.org/10.1016/j.athoracsur.2007.10.007.
Depypere L, Thomas M, Moons J, et al. Analysis of patients scheduled for neoadjuvant therapy followed by surgery for esophageal cancer, who never made it to esophagectomy. World J Surg Oncol. 2019;17(1):89. https://doi.org/10.1186/s12957-019-1630-8.
Arnett ALH, Merrell KW, Macintosh EM, et al. Utility of (18)F-FDG PET for predicting histopathologic response in esophageal carcinoma following chemoradiation. J Thorac Oncol. 2017;12(1):121–8. https://doi.org/10.1016/j.jtho.2016.08.136.
Elimova E, Wang X, Etchebehere E, et al. 18-fluorodeoxy-glucose positron emission computed tomography as predictive of response after chemoradiation in oesophageal cancer patients. Eur J Cancer. 2015;51(17):2545–52. https://doi.org/10.1016/j.ejca.2015.07.044.
Song SY, Kim JH, Ryu JS, et al. FDG-PET in the prediction of pathologic response after neoadjuvant chemoradiotherapy in locally advanced, resectable esophageal cancer. Int J Radiat Oncol Biol Phys. 2005;63(4):1053–9. https://doi.org/10.1016/j.ijrobp.2005.03.033.
Li Y, Zschaeck S, Lin Q, Chen S, Chen L, Wu H. Metabolic parameters of sequential 18F-FDG PET/CT predict overall survival of esophageal cancer patients treated with (chemo-) radiation. Radiat Oncol. 2019;14(1):35. https://doi.org/10.1186/s13014-019-1236-x.
Hyun SH, Ahn HK, Ahn MJ, et al. Volume-based assessment with 18F-FDG PET/CT improves outcome prediction for patients with stage IIIA-N2 non-small cell lung cancer. AJR Am J Roentgenol. 2015;205(3):623–8. https://doi.org/10.2214/ajr.14.13847.
Hamai Y, Hihara J, Emi M, et al. Ability of fluorine-18 fluorodeoxyglucose positron emission tomography to predict outcomes of neoadjuvant chemoradiotherapy followed by surgical treatment for esophageal squamous cell carcinoma. Ann Thorac Surg. 2016;102(4):1132–9. https://doi.org/10.1016/j.athoracsur.2016.04.011.
Blum Murphy M, Xiao L, Patel VR, et al. Pathological complete response in patients with esophageal cancer after the trimodality approach: the association with baseline variables and survival-The University of Texas MD Anderson Cancer Center experience. Cancer. 2017;123(21):4106–13. https://doi.org/10.1002/cncr.30953.
Kurokawa T, Hamai Y, Emi M, et al. Risk factors for recurrence in esophageal squamous cell carcinoma without pathological complete response after trimodal therapy. Anticancer Res. 2020;40(8):4387–94. https://doi.org/10.21873/anticanres.14442.
Khan M, Urooj N, Syed AA, et al. Prognostic factors for recurrence in esophageal cancer patients treated with neoadjuvant therapy and surgery: a single-institution analysis. Cureus. 2020;12(5): e8108. https://doi.org/10.7759/cureus.8108.
Tu CC, Hsu PK, Chien LI, et al. Prognostic histological factors in patients with esophageal squamous cell carcinoma after preoperative chemoradiation followed by surgery. BMC Cancer. 2017;17(1):62. https://doi.org/10.1186/s12885-017-3063-5.
Stahl M, Lehmann N, Walz MK, Stuschke M, Wilke H. Prediction of prognosis after trimodal therapy in patients with locally advanced squamous cell carcinoma of the oesophagus. Eur J Cancer. 2012;48(16):2977–82. https://doi.org/10.1016/j.ejca.2012.03.010.
Hamai Y, Hihara J, Emi M, et al. Evaluation of prognostic factors for esophageal squamous cell carcinoma treated with neoadjuvant chemoradiotherapy followed by surgery. World J Surg. 2018;42(5):1496–505. https://doi.org/10.1007/s00268-017-4283-1.
Lee HY, Hyun SH, Lee KS, et al. Volume-based parameter of 18)F-FDG PET/CT in malignant pleural mesothelioma: prediction of therapeutic response and prognostic implications. Ann Surg Oncol. 2010;17(10):2787–94. https://doi.org/10.1245/s10434-010-1107-z.
Shim SS, Lee KS, Kim BT, et al. Non-small cell lung cancer: prospective comparison of integrated FDG PET/CT and CT alone for preoperative staging. Radiology. 2005;236(3):1011–9. https://doi.org/10.1148/radiol.2363041310.
Lee JY, Kim YH, Park YJ, et al. Improved detection of metastatic lymph nodes in oesophageal squamous cell carcinoma by combined interpretation of fluorine-18-fluorodeoxyglucose positron-emission tomography/computed tomography. Cancer Imaging. 2019;19(1):40. https://doi.org/10.1186/s40644-019-0225-5.
Korst RJ, Rusch VW, Venkatraman E, et al. Proposed revision of the staging classification for esophageal cancer. J Thorac Cardiovasc Surg. 1998;115(3):660–669; discussion 669–670. https://doi.org/10.1016/s0022-5223(98)70332-0
Rice TW, Blackstone EH, Rusch VW. 7th edition of the AJCC Cancer Staging Manual: esophagus and esophagogastric junction. Ann Surg Oncol. 2010;17(7):1721–4. https://doi.org/10.1245/s10434-010-1024-1.
Mandard AM, Dalibard F, Mandard JC, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer. 1994;73(11):2680–6. https://doi.org/10.1002/1097-0142(19940601)73:11%3c2680::aid-cncr2820731105%3e3.0.co;2-c.
Kaplan ELMP. Nonparametric-estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457–81. https://doi.org/10.1080/01621459.1958.10501452.
Kock NLGS. Lateral collinearity and misleading results in variance-based SEM: an illustration and recommendations. J Assoc Inf Syst. 2012;13(7):546–80.
Schoenfeld DA. Partial residuals for the proportional hazards regression model. Biometrika. 1982;69:239–41.
Lin DY, Wei LJ, Ying Z. Checking the Cox model with cumulative sums of martingale-based residuals. Biometrika. 1993;80:557–72.
Miller RaS D. Maximally selected chi square statistics. Biometrics. 1982;38:1011–6.
Contal C, O’Quigley J. An application of changepoint methods in studying the effect of age on survival in breast cancer. Comput Stat Data Anal. 1999;30:253–70.
Shapiro J, van Lanschot JJB, Hulshof M, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16(9):1090–8. https://doi.org/10.1016/s1470-2045(15)00040-6.
Sjoquist KM, Burmeister BH, Smithers BM, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol. 2011;12(7):681–92. https://doi.org/10.1016/s1470-2045(11)70142-5.
van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366(22):2074–84. https://doi.org/10.1056/NEJMoa1112088.
Bedenne L, Michel P, Bouché O, et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol. 2007;25(10):1160–8. https://doi.org/10.1200/jco.2005.04.7118.
Stahl M, Stuschke M, Lehmann N, et al. Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol. 2005;23(10):2310–7. https://doi.org/10.1200/jco.2005.00.034.
Valkema MJ, Noordman BJ, Wijnhoven BPL, et al. Accuracy of (18)F-FDG PET/CT in predicting residual disease after neoadjuvant chemoradiotherapy for esophageal cancer. J Nucl Med. 2019;60(11):1553–9. https://doi.org/10.2967/jnumed.118.224196.
Hamai Y, Emi M, Ibuki Y, et al. Predictions of pathological features and recurrence based on FDG-PET Findings of esophageal squamous cell carcinoma after trimodal therapy. Ann Surg Oncol. 2020. https://doi.org/10.1245/s10434-020-08609-0.
Borggreve AS, Goense L, van Rossum PSN, et al. Preoperative prediction of pathologic response to neoadjuvant chemoradiotherapy in patients with esophageal cancer using (18)F-FDG PET/CT and DW-MRI: a prospective multicenter study. Int J Radiat Oncol Biol Phys. 2020;106(5):998–1009. https://doi.org/10.1016/j.ijrobp.2019.12.038.
Alnaji RM, Du W, Gabriel E, et al. Pathologic complete response is an independent predictor of improved survival following neoadjuvant chemoradiation for esophageal adenocarcinoma. J Gastrointest Surg. 2016;20(9):1541–6. https://doi.org/10.1007/s11605-016-3177-0.
Berger AC, Farma J, Scott WJ, et al. Complete response to neoadjuvant chemoradiotherapy in esophageal carcinoma is associated with significantly improved survival. J Clin Oncol. 2005;23(19):4330–7. https://doi.org/10.1200/jco.2005.05.017.
de Gouw D, Klarenbeek BR, Driessen M, et al. Detecting pathological complete response in esophageal cancer after neoadjuvant therapy based on imaging techniques: a diagnostic systematic review and meta-analysis. J Thorac Oncol. 2019;14(7):1156–71. https://doi.org/10.1016/j.jtho.2019.04.004.
Bedenne L. MC. Comparison of systematic surgery versus surveillance and rescue surgery in operable oesophageal cancer with a complete clinical response to radiochemotherapy (esostrate).https://clinicaltrials.gov/ct2/show/NCT02551458.
Noordman BJ, Wijnhoven BPL, Lagarde SM, et al. Neoadjuvant chemoradiotherapy plus surgery versus active surveillance for oesophageal cancer: a stepped-wedge cluster randomised trial. BMC Cancer. 2018;18(1):142. https://doi.org/10.1186/s12885-018-4034-1.
Guo JC, Huang TC, Lin CC, et al. Postchemoradiotherapy pathologic stage classified by the American Joint Committee on the Cancer Staging System predicts prognosis of patients with locally advanced esophageal squamous cell carcinoma. J Thorac Oncol. 2015;10(10):1481–9. https://doi.org/10.1097/jto.0000000000000651.
O JH, Jacene H, Luber B, et al. Quantitation of cancer treatment response by (18)F-FDG PET/CT: multicenter assessment of measurement variability. J Nucl Med. 2017;58(9):1429–1434. https://doi.org/10.2967/jnumed.117.189605
Acknowledgements
We are grateful for the librarians Myung-Ah Shim and Jaero Park for their dedicated support of manuscript formatting. Both librarians are working at the Samsung Medical Information & Media Services of Samsung Medical Center located in Seoul, South Korea.
Funding
This work was supported by the National R&D Program for Cancer Control, Ministry of Health & Welfare of Korea [1720180].
Author information
Authors and Affiliations
Contributions
Study conception and design: Yeonu Choi, Joon Young Choi, Hong Kwan Kim, Kyung Soo Lee. Data acquisition and analysis: Yeonu Choi, Joon Young Choi, Tae Hee Hong, Yoon-La Choi, Sook Young Woo, Kyung Soo Lee. Data interpretation and manuscript writing: Yeonu Choi, Joon Young Choi, Tae Hee Hong, Yoon-La Choi, Sook Young Woo, Kyung Soo Lee. Revision of manuscript and contribution of intellectual content: All authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yeonu Choi and Joon Young Choi contributed equally to this manuscript.
This article is part of the Topical Collection on Oncology - Digestive tract
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Choi, Y., Choi, J.Y., Hong, T.H. et al. Trimodality therapy for locally advanced esophageal squamous cell carcinoma: the role of volume-based PET/CT in patient management and prognostication. Eur J Nucl Med Mol Imaging 49, 751–762 (2022). https://doi.org/10.1007/s00259-021-05487-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-021-05487-w