Abstract
Purpose
The conventional imaging flowchart for prostate cancer (PCa) staging may fail in correctly detecting lymph node metastases (LNM). Pelvic lymph node dissection (PLND) represents the only reliable method, although invasive. A new amino acid PET compound, [18F]-fluciclovine, was recently authorized in suspected PCa recurrence but not yet included in the standard staging work-up of primary PCa. A prospective monocentric study was designed to evaluate [18F]-fluciclovine PET/CT diagnostic performance for preoperative LN staging in primary high-risk PCa.
Methods
Consecutive patients (pts) with biopsy-proven PCa, standard staging (including [11C]choline PET/CT), eligible for PLND, were enrolled to undergo an investigational [18F]-fluciclovine PET/CT. Nodal uptake higher than surrounding background was reported by at least two readers (blinded to [11C]choline) using a visual 5-point scale (1–2 probably negative; 4–5 probably positive; 3 equivocal); SUVmax, target-to-background (aorta—A; bone marrow—BM) ratios (TBRs), were also calculated. PET results were validated with PLND. [18F]-fluciclovine PET/CT performance using visual score and semi-quantitative indexes was analyzed both per patient and per LN anatomical region, compared to conventional [11C]choline and clinical predictive factors (to note that diagnostic performance of [18F]-fluciclovine was explored for LNM but not examined for intrapelvic or extrapelvic M1 lesions).
Results
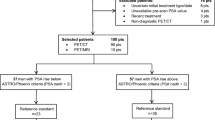
Overall, 94 pts underwent [18F]-fluciclovine PET/CT; 72/94 (77%) high-risk pts were included in the final analyses (22 pts excluded: 8 limited PLND; 3 intermediate-risk; 2 treated with radiotherapy; 4 found to be M1; 5 neoadjuvant hormonal therapy). Median LNM risk by Briganti nomogram was 19%. LNM confirmed on histology was 25% (18/72 pts). Overall, 1671 LN were retrieved; 45/1671 (3%) LNM detected. Per pt, median no. of removed LN was 22 (mean 23 ± 10; range 8–51), of LNM was 2 (mean 3 ± 2; range 1–10). Median LNM size was 5 mm (mean 5 ± 2.5; range 2–10). On patient-based analyses (n = 72), diagnostic performance for LNM resulted significant with [18F]-fluciclovine (AUC 0.66, p 0.04; 50% sensitivity, 81% specificity, 47% PPV, 83% NPV, 74% accuracy), but not with [11C]choline (AUC 0.60, p 0.2; 50%, 70%, 36%, 81%, and 65% respectively). Briganti nomogram (OR = 1.03, p = 0.04) and [18F]-fluciclovine visual score (≥ 4) (OR = 4.27, p = 0.02) resulted independent predictors of LNM at multivariable analyses. On region-based semi-quantitative analyses (n = 576), PET/CT performed better using TBR parameters (TBR-A similar to TBR-BM; TBR-A fluciclovine AUC 0.61, p 0.35, vs choline AUC 0.57 p 0.54; TBR-BM fluciclovine AUC 0.61, p 0.36, vs choline AUC 0.58, p 0.52) rather than using absolute LN SUVmax (fluciclovine AUC 0.51, p 0.91, vs choline AUC 0.51, p 0.94). However, in all cases, diagnostic performance was not statistically significant for LNM detection, although slightly in favor of the experimental tracer [18F]-fluciclovine for each parameter. On the contrary, visual interpretation significantly outperformed PET semi-quantitative parameters (choline and fluciclovine: AUC 0.65 and 0.64 respectively; p 0.03) and represents an independent predictive factor of LNM with both tracers, in particular [18F]-fluciclovine (OR = 8.70, p 0.002, vs OR = 3.98, p = 0.03).
Conclusion
In high-risk primary PCa, [18F]-fluciclovine demonstrates some advantages compared with [11C]choline but sensitivity for metastatic LN detection is still inadequate compared to PLND. Visual (combined morphological and functional), compared to semi-quantitative assessment, is promising but relies mainly on readers’ experience rather than on unquestionable LN avidity.
Trial registration
EudraCT number: 2014–003,165-15




Similar content being viewed by others
References
EAU Guidelines: Prostate cancer | Uroweb [Internet]. [cited 2020 Nov 30]. Available from: https://uroweb.org/guideline/prostate-cancer/
Mottet N, Bellmunt J, Briers E, Bergh RCN van den, Bolla M, Casteren NJ van, et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer-2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur Urol. 2021;79(2):243–262.
Testa C, Schiavina R, Lodi R, Salizzoni E, Tonon C, D’Errico A, et al. Accuracy of MRI/MRSI-based transrectal ultrasound biopsy in peripheral and transition zones of the prostate gland in patients with prior negative biopsy. NMR Biomed NMR Biomed. 2010;23:1017–26.
Schiavina R, Bianchi L, Borghesi M, Dababneh H, Chessa F, Pultrone CV, et al. MRI displays the prostatic cancer anatomy and improves the bundles management before robot-assisted radical prostatectomy. J Endourol Mary Ann Liebert Inc. 2018;32:315–21.
Schiavina R, Bertaccini A, Franceschelli A, Manferrari F, Vagnoni V, Borghesi M, et al. The impact of the extent of lymph-node dissection on biochemical relapse after radical prostatectomy in node-negative patients. Anticancer Res. 2010;30(6):2297–302.
Ganeshalingam S, Koh D-M. ARTICLE Nodal staging. Cancer Imaging. 2009;9:104–11.
Ceci F, Castellucci P, Mamede M, Schiavina R, Rubello D, Fuccio C, et al. 11C-Choline PET/CT in patients with hormone-resistant prostate cancer showing biochemical relapse after radical prostatectomy Eur J Nucl Med Mol Imaging. Eur J Nucl Med Mol Imaging. 2013;40:149–55.
Schiavina R, Chessa F, Borghesi M, Gaudiano C, Bianchi L, Corcioni B, et al. State-of-the-art imaging techniques in the management of preoperative staging and re-staging of prostate cancer. Int. J. Urol. Blackwell Publishing; 2019. p. 18–30.
Schiavina R, Bianchi L, Mineo Bianchi F, Borghesi M, Pultrone CV, Dababneh H, et al. Preoperative staging with 11C-choline PET/CT is adequately accurate in patients with very high-risk prostate cancer. Clin Genitourin Cancer Elsevier Inc. 2018;16:305-312.e1.
Thoeny HC, Barbieri S, Froehlich JM, Turkbey B, Choyke PL. Functional and targeted lymph node imaging in prostate cancer: current status and future challenges. Radiology. Radiological Society of North America Inc.; 2017. p. 728–43.
Weckermann D, Dorn R, Holl G, Wagner T, Harzmann R. Limitations of radioguided surgery in high-risk prostate cancer. Eur Urol Eur Urol. 2007;51:1549–58.
Farolfi A Ceci F Castellucci P Graziani T Siepe G Lambertini A et al 68Ga-PSMA-11 PET/CT in prostate cancer patients with biochemical recurrence after radical prostatectomy and PSA <0.5 ng/ml Efficacy and impact on treatment strategy Eur J Nucl Med Mol Imaging Springer Berlin Heidelberg 2019 4611 9
Ceci F, Bianchi L, Borghesi M, Polverari G, Farolfi A, Briganti A, et al. Prediction nomogram for 68Ga-PSMA-11 PET/CT in different clinical settings of PSA failure after radical treatment for prostate cancer. Eur J Nucl Med Mol Imaging Springer. 2020;47:136–46.
Bianchi L, Schiavina R, Borghesi M, Ceci F, Angiolini A, Chessa F, et al. How does 68Ga-prostate-specific membrane antigen positron emission tomography/computed tomography impact the management of patients with prostate cancer recurrence after surgery? Int J Urol Blackwell Publishing. 2019;26:804–11.
Fendler WP, Eiber M, Beheshti M, Bomanji J, Ceci F, Cho S, et al. 68Ga-PSMA PET/CT: Joint EANM and SNMMI procedure guideline for prostate cancer imaging: version 1.0. Eur J Nucl Med Mol Imaging. Springer Berlin; 2017;44:1014–24
Briganti A, Larcher A, Abdollah F, Capitanio U, Gallina A, Suardi N, et al. Updated nomogram predicting lymph node invasion in patients with prostate cancer undergoing extended pelvic lymph node dissection: the essential importance of percentage of positive cores. Eur Urol Eur Urol. 2012;61:480–7.
Schuster DM, Votaw JR, Nieh PT, Yu W, Nye JA, Master V, et al. Initial experience with the radiotracer anti-1-amino-3-18F- fluorocyclobutane-1-carboxylic acid with PET/CT in prostate carcinoma. J Nucl Med. 2007;48:56–63.
Oka S, Hattori R, Kurosaki F, Toyama M, Williams LA, Yu W, et al. A preliminary study of anti-1-amino-3–18F-fluorocyclobutyl-1-carboxylic acid for the detection of prostate cancer. J Nucl Med. 2007;48.
Nye JA, Schuster DM, Yu W, Camp VM, Goodman MM, Votaw JR. Biodistribution and radiation dosimetry of the synthetic nonmetabolized amino acid analogue anti-18F-FACBC in humans. J Nucl Med J Nucl Med. 2007;48:1017–20.
Sörensen J, Owenius R, Lax M, Johansson S. Regional distribution and kinetics of [ 18 F]fluciclovine (anti-[ 18 F]FACBC), a tracer of amino acid transport, in subjects with primary prostate cancer.
Schuster DM, Taleghani PA, Nieh PT, Master VA, Amzat R, Savir-Baruch B, et al. Characterization of primary prostate carcinoma by anti-1-amino-2-[(18)F] -fluorocyclobutane-1-carboxylic acid (anti-3-[(18)F] FACBC) uptake. Am J Nucl Med Mol Imaging e-Century Publishing Corporation. 2013;3:85–96.
Nanni C, Zanoni L, Pultrone C, Schiavina R, Brunocilla E, Lodi F, et al. 18F-FACBC (anti1-amino-3–18F-fluorocyclobutane-1-carboxylic acid) versus 11C-choline PET/CT in prostate cancer relapse: results of a prospective trial. Eur J Nucl Med Mol Imaging. 2016;43.
Nanni C, Schiavina R, Brunocilla E, Borghesi M, Ambrosini V, Zanoni L, et al. 18F-FACBC compared with 11C-choline PET/CT in patients with biochemical relapse after radical prostatectomy: A prospective study in 28 patients. Clin Genitourin Cancer. 2014;12.
Nanni C, Schiavina R, Brunocilla E, Boschi S, Borghesi M, Zanoni L, et al. 18F-Fluciclovine PET/CT for the detection of prostate cancer relapse: a comparison to 11C-Choline PET/CT. Clin Nucl Med. 2015;40.
Zanoni L, Bossert I, Matti A, Schiavina R, Pultrone C, Fanti S, et al. A review discussing fluciclovine (18F) PET/CT imaging in the detection of recurrent prostate cancer. Futur Oncol. 2018;14.
Bach-Gansmo T, Nanni C, Nieh PT, Zanoni L, Bogsrud TV, Sletten H, et al. Multisite experience of the safety, detection rate and diagnostic performance of fluciclovine ( 18 F) positron emission tomography/computerized tomography imaging in the staging of biochemically recurrent prostate cancer. J Urol. 2017;197.
FDA approves new diagnostic imaging agent to detect recurrent prostate cancer | FDA [Internet]. [cited 2021 Feb 7]. Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-new-diagnostic-imaging-agent-detect-recurrent-prostate-cancer
Axumin | European Medicines Agency [Internet]. [cited 2021 Feb 7]. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/axumin
Schuster DM, Votaw JR, Nieh PT, Yu W, Nye JA, Master V, et al. Initial experience with the radiotracer anti-1-amino-3–18 F-fluorocyclobutane-1-carboxylic acid with PET/CT in prostate carcinoma.
European Pharmacopoeia (Ph. Eur.) 10th Edition | EDQM - European Directorate for the Quality of Medicines [Internet]. [cited 2021 Feb 7]. Available from: https://www.edqm.eu/en/european-pharmacopoeia-ph-eur-10th-edition
AIMN - linee-guida [Internet]. [cited 2021 Feb 7]. Available from: https://www.aimn.it/site/page/attivita/linee-guida
Bianchi L, Schiavina R, Borghesi M, Casablanca C, Chessa F, Bianchi FM, et al. Patterns of positive surgical margins after open radical prostatectomy and their association with clinical recurrence. Minerva Urol e Nefrol Edizioni Minerva Medica. 2020;72:464–73.
Bianchi L, Gandaglia G, Fossati N, Suardi N, Moschini M, Cucchiara V, et al. Pelvic lymph node dissection in prostate cancer: indications, extent and tailored approaches. Urologia. Urologia; 2017. p. 9–19.
Kanagawa M, Doi Y, Oka S, Kobayashi R, Nakata N, Toyama M, et al. Comparison of trans-1-amino-3-[18F]fluorocyclobutanecarboxylic acid (anti-[18F]FACBC) accumulation in lymph node prostate cancer metastasis and lymphadenitis in rats. Nucl Med Biol Elsevier Inc. 2014;41:545–51.
Suzuki H, Jinnouchi S, Kaji Y, Kishida T, Kinoshita H, Yamaguchi S, et al. Diagnostic performance of 18F-fluciclovine PET/CT for regional lymph node metastases in patients with primary prostate cancer: a multicenter phase II clinical trial. Jpn J Clin Oncol Oxford University Press. 2019;49:803–11.
Kim SJ, Lee SW. The role of 18F-fluciclovine PET in the management of prostate cancer: a systematic review and meta-analysis. Clin Radiol WB Saunders Ltd. 2019;74:886–92.
Hoekstra RJ, Beulens A, Vrijhof EHJEJ, Wyndaele DNJ, Roef M, Brouwer LJM, et al. Diagnostic accuracy of 18F-fluciclovine PET/CT in primary lymph node staging of prostate cancer. Nucl Med Commun. 2021;42(5):476–481.
Alemozaffar M, Akintayo AA, Abiodun-Ojo OA, Patil D, Saeed F, Huang Y, et al. 18F]Fluciclovine positron emission tomography/computerized tomography for preoperative staging in patients with intermediate to high risk primary prostate cancer. J Urol NLM (Medline). 2020;204:734–40.
Selnæs KM, Krüger-Stokke B, Elschot M, Willoch F, Størkersen Ø, Sandsmark E, et al. 18F-Fluciclovine PET/MRI for preoperative lymph node staging in high-risk prostate cancer patients. Eur Radiol Springer Verlag. 2018;28:3151–9.
Jambor I, Kuisma A, Kähkönen E, Kemppainen J, Merisaari H, Eskola O, et al. Prospective evaluation of 18 F-FACBC PET/CT and PET/MRI versus multiparametric MRI in intermediate-to high-risk prostate cancer patients (FLUCIPRO trial). Eur J Nucl Med Mol Imaging. 2018;45:355–64.
Galgano SJ, Mcdonald A, Rais-Bahrami S, Porter KK, Choudhary G, Burgan C, et al. Utility of 18F-fluciclovine PET/MRI for staging newly diagnosed high-risk prostate cancer and evaluating response to initial androgen deprivation therapy: a prospective single-arm pilot study.AJR Am J Roentgenol. 2020 Oct 14.
Von Eyben FE, Kairemo K. Meta-analysis of 11C-choline and 18F-choline PET/CT for management of patients with prostate cancer. Nucl. Med. Commun. Nucl Med Commun; 2014. p. 221–30.
Evangelista L, Zattoni F, Karnes RJ, Novara G, Lowe V. Radiolabeled choline PET/CT before salvage lymphadenectomy dissection: a systematic review and meta-analysis. Nucl. Med. Commun. Lippincott Williams and Wilkins; 2016. p. 1223–31.
Evangelista L, Briganti A, Fanti S, Joniau S, Reske S, Schiavina R, et al. New clinical indications for 18F/11C-choline, new tracers for positron emission tomography and a promising hybrid device for prostate cancer staging: a systematic review of the literature [Figure presented]. Eur. Urol. Elsevier B.V.; 2016. p. 161–75.
Harisinghani MG, Barentsz J, Hahn PF, Deserno WM, Tabatabaei S, van de Kaa CH, et al. Noninvasive detection of clinically occult lymph-node metastases in prostate cancer. N Engl J Med New England Journal of Medicine (NEJM/MMS). 2003;348:2491–9.
Hövels AM, Heesakkers RAM, Adang EM, Jager GJ, Strum S, Hoogeveen YL, et al. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: a meta-analysis. Clin Radiol Clin Radiol. 2008;63:387–95.
Gabriele D, Collura D, Oderda M, Stura I, Fiorito C, Porpiglia F, et al. Is there still a role for computed tomography and bone scintigraphy in prostate cancer staging? An analysis from the EUREKA-1 database. World J Urol Springer Verlag. 2016;34:517–23.
Flanigan RC, McKay TC, Olson M, Shankey TV, Pyle J, Waters WB. Limited efficacy of preoperative computed tomographic scanning for the evaluation of lymph node metastasis in patients before radical prostatectomy. Urology. Elsevier Inc. 1996;48:428–32.
Tiguert R, Gheiler EL, Tefilli MV, Oskanian P, Banerjee M, Grignon DJ, et al. Lymph node size does not correlate with the presence of prostate cancer metastasis. Urology Urology. 1999;53:367–71.
Spevack L, Killion LT, West JC, Rooker GM, Brewer EA, Cuddy PG. Predicting the patient at low risk for lymph node metastasis with localized prostate cancer: an analysis of four statistical models. Int J Radiat Oncol Biol Phys Elsevier Inc. 1996;34:543–7.
Nanni C, Zanoni L, Bach-Gansmo T, Minn H, Willoch F, Bogsrud TV, et al. [18F]Fluciclovine PET/CT: joint EANM and SNMMI procedure guideline for prostate cancer imaging—version 1.0. Eur J Nucl Med Mol Imaging. 2020;47.
Schiavina R, Scattoni V, Castellucci P, Picchio M, Corti B, Briganti A, et al. 11C-Choline positron emission tomography/computerized tomography for preoperative lymph-node staging in intermediate-risk and high-risk prostate cancer: comparison with clinical staging nomograms. Eur Urol Eur Urol. 2008;54:392–401.
Miller MP, Kostakoglu L, Pryma D, Yu JQ, Chau A, Perlman E, et al. Reader training for the restaging of biochemically recurrent prostate cancer using 18F-fluciclovine PET/CT. J Nucl Med Society of Nuclear Medicine Inc. 2017;58:1596–602.
McDonald AM, Galgano SJ, McConathy JE, Yang ES, Dobelbower MC, Jacob R, et al. Feasibility of dose escalating [18F]fluciclovine positron emission tomography positive pelvic lymph nodes during moderately hypofractionated radiation therapy for high-risk prostate cancer. Adv Radiat Oncol Elsevier Inc. 2019;4:649–58.
Schiavina R, Capizzi E, Borghesi M, Vagnoni V, Romagnoli D, Rocca GC, et al. Nodal occult metastases in intermediate- and high-risk prostate cancer patients detected using serial section, immunohistochemistry, and real-time reverse transcriptase polymerase chain reaction: Prospective evaluation with matched-pair analysis. Clin Genitourin Cancer Elsevier Inc. 2015;13:e55–64.
Borrelli P, Larsson M, Ulén J, Enqvist O, Trägårdh E, Poulsen MH, et al. Artificial intelligence-based detection of lymph node metastases by PET/CT predicts prostate cancer-specific survival. Clin Physiol Funct Imaging Blackwell Publishing Ltd. 2021;41:62–7.
Maurer T, Gschwend JE, Rauscher I, Souvatzoglou M, Haller B, Weirich G, et al. Diagnostic efficacy of 68Gallium-PSMA positron emission tomography compared to conventional imaging for lymph node staging of 130 consecutive patients with intermediate to high risk prostate cancer. J Urol Elsevier Inc. 2016;195:1436–43.
Herlemann A, Wenter V, Kretschmer A, Thierfelder KM, Bartenstein P, Faber C, et al. 68Ga-PSMA positron emission tomography/computed tomography provides accurate staging of lymph node regions prior to lymph node dissection in patients with prostate cancer. Eur Urol Elsevier BV. 2016;70:553–7.
Hijazi S, Meller B, Leitsmann C, Strauss A, Meller J, Ritter CO, et al. Pelvic lymph node dissection for nodal oligometastatic prostate cancer detected by 68Ga-PSMA-positron emission tomography/computerized tomography. Prostate John Wiley and Sons Inc. 2015;75:1934–40.
Pyka T, Okamoto S, Dahlbender M, Tauber R, Retz M, Heck M, et al. Comparison of bone scintigraphy and 68Ga-PSMA PET for skeletal staging in prostate cancer. Eur J Nucl Med Mol Imaging Springer Berlin. 2016;43:2114–21.
Perera M, Papa N, Roberts M, Williams M, Udovicich C, Vela I, et al. Gallium-68 prostate-specific membrane antigen positron emission tomography in advanced prostate cancer—updated diagnostic utility, sensitivity, specificity, and distribution of prostate-specific membrane antigen-avid lesions: a systematic review and meta-analysis. Eur. Urol. Elsevier B.V.; 2020. p. 403–17.
van Kalmthout LWM, van Melick HHE, Lavalaye J, Meijer RP, Kooistra A, de Klerk JMH, et al. Prospective validation of gallium-68 prostate specific membrane antigen-positron emission tomography/computerized tomography for primary staging of prostate cancer. J Urol NLM (Medline). 2020;203:537–45.
Wu H, Xu T, Wang X, Yu YB, Fan ZY, Li DX, et al. Diagnostic performance of 68gallium labelled prostate-specific membrane antigen positron emission tomography/computed tomography and magnetic resonance imaging for staging the prostate cancer with intermediate or high risk prior to radical prostatectomy: a systematic review and meta-analysis. World J. Mens. Health. Korean Society for Sexual Medicine and Andrology; 2020. p. 208–19.
Tulsyan S, Das CJ, Tripathi M, Seth A, Kumar R, Bal C. Comparison of 68 Ga-PSMA PET/CT and multiparametric MRI for staging of high-risk prostate cancer 68 Ga-PSMA PET and MRI in prostate cancer. Nucl Med Commun Lippincott Williams and Wilkins. 2017;38:1094–102.
Uprimny C, Kroiss AS, Decristoforo C, Fritz J, von Guggenberg E, Kendler D, et al. 68Ga-PSMA-11 PET/CT in primary staging of prostate cancer: PSA and Gleason score predict the intensity of tracer accumulation in the primary tumour. Eur J Nucl Med Mol Imaging Springer Berlin. 2017;44:941–9.
Budäus L, Leyh-Bannurah SR, Salomon G, Michl U, Heinzer H, Huland H, et al. Initial experience of 68Ga-PSMA PET/CT imaging in high-risk prostate cancer patients prior to radical prostatectomy. Eur Urol Elsevier BV. 2016;69:393–6.
Schuster DM, Nanni C, Fanti S, Oka S, Okudaira H, Inoue Y, et al. Anti-1-amino-3–18F-fluorocyclobutane-1-carboxylic acid: physiologic uptake patterns, incidental findings, and variants that may simulate disease. J Nucl Med Society of Nuclear Medicine Inc. 2014;55:1986–92.
Giesel FL, Sterzing F, Schlemmer HP, Holland-Letz T, Mier & W, Rius & M, et al. Intra-individual comparison of 68 Ga-PSMA-11-PET/CT and multi-parametric MR for imaging of primary prostate cancer. Eur J Nucl Med Mol Imaging. 2016;43(8):1400–6.
Acknowledgements
Blue Earth Diagnostics (BED) kindly provided pre-loaded cassettes for [18F]-fluciclovine synthesis, thanks to an agreement without any financial relationship.
Funding
This research was funded by “programma di Ricerca Regione-Università Area 1 Bando Giovani Ricercatori “Alessandro Liberati 2013,” grant number PRUA1GR-2013–00000171.
Author information
Authors and Affiliations
Contributions
Conceptualization: ZL, NC, FS, SR, FM, and DEA; methodology: ZL, FS, and FC; formal analysis: ZL, BL, and FC; investigation: ZL, BI, MA, PC, GF, FM, SR, BL, NC, PA, and RD; resources: LF; data curation: ZL, BI, MA, GF, PC, BL, FC, and RD; writing—original draft preparation: ZL and BL; writing—review and editing: all authors; visualization: ZL and BL; supervision: ZL, NC, FS, SR, BE, MF, PA, and DEA; project administration: ZL and FS; funding acquisition: ZL, FS, FC, and NC. All authors have read and agreed to the published version of the manuscript and contributed substantially to the work reported. In particular, in order to qualify for authorship of a manuscript, the following criteria were observed: Substantial contributions to the conception or design of the work, or the acquisition, analysis, or interpretation of data for the work; AND Drafting the work or revising it critically for important intellectual content; AND Final approval of the version to be published; AND Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the Local Bioethics Committee (EudraCT number 2014–003165-15; Local Ethical Committee code: 139/2014/O/Sper).
Consent to participate and consent for publication
All patients included in the study signed informed consent to participate and publication. Informed consent to participate and for publication was obtained from all individual participants included in the study.
Competing interests
Lucia Zanoni had a scientific-only relationship with the company “Blue Earth Diagnostics Ltd.” as Medical Staff of the Sponsored Study BED001 (118/2014/O/Oss) (no financial relationship, no compensation received). She was Principal Investigator of the project entitled “18F-FACBC PET/CT for staging high risk prostate cancer” funded by “Programma di ricercar Regione-Università 2013-Area 1 “Ricerca Innovativa,” Bando “Alessandro Liberati-Giovani Ricercatori.” In the context of this project, Lucia Zanoni received a granted 1-year SSN contract as nuclear medicine project manager (both scientific and financial relationship; 2016). Cristina Nanni provided consultancy for Blue Earth Diagnostics Ltd. 2018–1019. She was Principal Investigator of the project entitled ANTI-3-18F-FACBC (anti1-amino-3-18F-fluorocyclobutane-1-carboxylic acid) in comparison to [11C]choline PET/CT in the evaluation of patients with prostate cancer radically treated and with rising PSA, “Programma di ricerca Regione-Università 2010–2012-Area 1” “Ricerca Innovativa,” Bando “Alessandro Liberati-Giovani Ricercatori.” Irene Bossert, Cristina Fonti, Cristian Pultrone, and Francesca Giunchi received granted universitary contracts as Staff of the project entitled “18F-FACBC PET/CT for staging high risk prostate cancer” funded by “Programma di ricerca Regione-Università 2013-Area 1” “Ricerca Innovativa,” Bando “Alessandro Liberati-Giovani Ricercatori.” Stefano Fanti, with respect to the mentioned paper, declares that he attended an advisory board of Blue Earth Diagnostics in 2014 and in 2015. The other authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Oncology - Genitourinary
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zanoni, L., Bianchi, L., Nanni, C. et al. [18F]-Fluciclovine PET/CT for preoperative nodal staging in high-risk primary prostate cancer: final results of a prospective trial. Eur J Nucl Med Mol Imaging 49, 390–409 (2021). https://doi.org/10.1007/s00259-021-05429-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-021-05429-6