Abstract
Purpose
18F-Fluciclovine PET imaging has been increasingly used in the restaging of prostate cancer patients with biochemical recurrence (BCR); however, its clinical utility in patients with low prostate-specific antigen (PSA) levels following primary radiation therapy has not been well-studied. This study aims to determine the detection rate and diagnostic accuracy of 18F-fluciclovine PET and the patterns of prostate cancer recurrence in patients with rising PSA after initial radiation therapy, particularly in patients with PSA levels below the accepted Phoenix definition of BCR (PSA nadir +2 ng/mL).
Methods
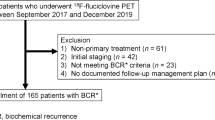
This retrospective study included patients from two tertiary institutions who underwent 18F-fluciclovine PET scans for elevated PSA level following initial external beam radiation therapy, brachytherapy, and/or proton therapy. Logistic regression and receiver operating characteristic (ROC) curve analyses were performed to determine the diagnostic accuracy of 18F-fluciclovine PET and associations of PSA kinetic parameters with 18F-fluciclovine PET outcome.
Results
One hundred patients were included in this study. The overall detection rate on a patient-level was 79% (79/100). 18F-Fluciclovine PET was positive in 62% (23/37) of cases with PSA below the Phoenix criteria. The positive predictive value of 18F-fluciclovine PET was 89% (95% CI: 80–94%). In patients with PSA below the Phoenix criteria, the PSA velocity had the highest predictive value of 18F-fluciclovine PET outcome. PSA doubling time (PSADT) and PSA velocity were associated with the presence of extra-pelvic metastatic disease.
Conclusion
18F-Fluciclovine PET can identify recurrent disease at low PSA level and PSA rise below accepted Phoenix criteria in patients with suspected BCR after primary radiation therapy, particularly in patients with low PSADT or high PSA velocity. In patients with low PSADT or high PSA velocity, there is an increased probability of extra-pelvic metastases. Therefore, these patients are more likely to benefit from PET/CT or PET/MRI than pelvic MRI alone.



Similar content being viewed by others
Data availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30.
Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271–89.
Zumsteg ZS, Spratt DE, Romesser PB, Pei X, Zhang Z, Polkinghorn W, et al. The natural history and predictors of outcome following biochemical relapse in the dose escalation era for prostate cancer patients undergoing definitive external beam radiotherapy. Eur Urol. 2015;67:1009–16.
Roach M 3rd, Hanks G, Thames H Jr, Schellhammer P, Shipley WU, Sokol GH, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65:965–74.
Tan N, Oyoyo U, Bavadian N, Ferguson N, Mukkamala A, Calais J, et al. PSMA-targeted radiotracers versus (18)F fluciclovine for the detection of prostate cancer biochemical recurrence after definitive therapy: a systematic review and meta-analysis. Radiology. 2020;296:44–55.
Turkbey B, Mena E, Shih J, Pinto PA, Merino MJ, Lindenberg ML, et al. Localized prostate cancer detection with 18F FACBC PET/CT: comparison with MR imaging and histopathologic analysis. Radiology. 2014;270:849–56.
Alemozaffar M, Akintayo AA, Abiodun-Ojo OA, Patil D, Saeed F, Huang Y, et al. [(18)F]Fluciclovine positron emission tomography/computerized tomography for preoperative staging in patients with intermediate to high risk primary prostate cancer. J Urol. 2020;204:734–40.
Nanni C, Zanoni L, Pultrone C, Schiavina R, Brunocilla E, Lodi F, et al. (18)F-FACBC (anti1-amino-3-(18)F-fluorocyclobutane-1-carboxylic acid) versus (11)C-choline PET/CT in prostate cancer relapse: results of a prospective trial. Eur J Nucl Med Mol Imaging. 2016;43:1601–10.
Akin-Akintayo OO, Jani AB, Odewole O, Tade FI, Nieh PT, Master VA, et al. Change in salvage radiotherapy management based on guidance with FACBC (fluciclovine) PET/CT in postprostatectomy recurrent prostate cancer. Clin Nucl Med. 2017;42:e22–e8.
Solanki AA, Savir-Baruch B, Liauw SL, Michalski J, Tward JD, Vapiwala N, et al. (18)F-Fluciclovine positron emission tomography in men with biochemical recurrence of prostate cancer after radical prostatectomy and planning to undergo salvage radiation therapy: results from LOCATE. Pract Radiat Oncol. 2020;10:354–62.
Calais J, Ceci F, Eiber M, Hope TA, Hofman MS, Rischpler C, et al. (18)F-fluciclovine PET-CT and (68)Ga-PSMA-11 PET-CT in patients with early biochemical recurrence after prostatectomy: a prospective, single-centre, single-arm, comparative imaging trial. Lancet Oncol. 2019;20:1286–94.
Odewole OA, Tade FI, Nieh PT, Savir-Baruch B, Jani AB, Master VA, et al. Recurrent prostate cancer detection with anti-3-[(18)F]FACBC PET/CT: comparison with CT. Eur J Nucl Med Mol Imaging. 2016;43:1773–83.
Bach-Gansmo T, Nanni C, Nieh PT, Zanoni L, Bogsrud TV, Sletten H, et al. Multisite experience of the safety, detection rate and diagnostic performance of fluciclovine ((18)F) positron emission tomography/computerized tomography imaging in the staging of biochemically recurrent prostate cancer. J Urol. 2017;197:676–83.
Andriole GL, Kostakoglu L, Chau A, Duan F, Mahmood U, Mankoff DA, et al. The impact of positron emission tomography with 18F-fluciclovine on the treatment of biochemical recurrence of prostate Cancer: results from the LOCATE trial. J Urol. 2019;201:322–31.
England JR, Paluch J, Ballas LK, Jadvar H. 18F-Fluciclovine PET/CT detection of recurrent prostate carcinoma in patients with serum PSA </= 1 ng/mL after definitive primary treatment. Clin Nucl Med. 2019;44:e128–e32.
Savir-Baruch B, Lovrec P, Solanki AA, Adams WH, Yonover PM, Gupta G, et al. Fluorine-18-labeled fluciclovine PET/CT in clinical practice: factors affecting the rate of detection of recurrent prostate cancer. AJR Am J Roentgenol. 2019;213:851–8.
Schuster DM, Nieh PT, Jani AB, Amzat R, Bowman FD, Halkar RK, et al. Anti-3-[(18)F]FACBC positron emission tomography-computerized tomography and (111)In-capromab pendetide single photon emission computerized tomography-computerized tomography for recurrent prostate carcinoma: results of a prospective clinical trial. J Urol. 2014;191:1446–53.
Schuster DM, Savir-Baruch B, Nieh PT, Master VA, Halkar RK, Rossi PJ, et al. Detection of recurrent prostate carcinoma with anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid PET/CT and 111In-capromab pendetide SPECT/CT. Radiology. 2011;259:852–61.
Akin-Akintayo O, Tade F, Mittal P, Moreno C, Nieh PT, Rossi P, et al. Prospective evaluation of fluciclovine ((18)F) PET-CT and MRI in detection of recurrent prostate cancer in non-prostatectomy patients. Eur J Radiol. 2018;102:1–8.
Savir-Baruch B, Banks KP, McConathy JE, Molchanova-Cook OP, Parent EE, Takalkar A, et al. ACR-ACNM practice parameter for the performance of fluorine-18 fluciclovine-PET/CT for recurrent prostate cancer. Clin Nucl Med. 2018;43:909–17.
Fendler WP, Calais J, Eiber M, Flavell RR, Mishoe A, Feng FY, et al. Assessment of 68Ga-PSMA-11 PET accuracy in localizing recurrent prostate cancer: a prospective single-arm clinical trial. JAMA Oncol. 2019;5:856–63.
Available from: https://www.mskcc.org/nomograms/prostate/psa_doubling_tim. Accessed between July 2019 - December 2020.
Trock BJ, Han M, Freedland SJ, Humphreys EB, DeWeese TL, Partin AW, et al. Prostate cancer-specific survival following salvage radiotherapy vs observation in men with biochemical recurrence after radical prostatectomy. JAMA. 2008;299:2760–9.
Lawhn-Heath C, Salavati A, Behr SC, Rowe SP, Calais J, Fendler WP, et al. Prostate-specific membrane antigen PET in prostate cancer. Radiology. 2021;299:248-260
Fendler WP, Calais J, Eiber M, Simko JP, Kurhanewicz J, Santos RD, et al. False positive PSMA PET for tumor remnants in the irradiated prostate and other interpretation pitfalls in a prospective multi-center trial. Eur J Nucl Med Mol Imaging. 2021;48:501–8.
Siddiqui MM, Rais-Bahrami S, Turkbey B, George AK, Rothwax J, Shakir N, et al. Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA. 2015;313:390–7.
Raveenthiran S, Yaxley J, Gianduzzo T, Kua B, McEwan L, Wong D, et al. The use of (68)Ga-PET/CT PSMA to determine patterns of disease for biochemically recurrent prostate cancer following primary radiotherapy. Prostate Cancer Prostatic Dis. 2019;22:385–90.
Sonni I, Eiber M, Fendler WP, Alano RM, Vangala SS, Kishan AU, et al. Impact of (68)Ga-PSMA-11 PET/CT on staging and management of prostate cancer patients in various clinical settings: a prospective single-center study. J Nucl Med. 2020;61:1153–60.
Code availability
Not applicable.
Funding
Ali Salavati was supported by the Radiological Society of North America (RSNA) Research & Education Foundation, through grant number RR1963.The content is solely the responsibility of the authors and does notnecessarily represent the official views of the RSNA R&E Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
This retrospective chart review study involving human participants was in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The Human Investigation Committee (IRB) of University of California, San Francisco, and University of Minnesota approved this study.
Consent to participate
Informed consent was waived by the local Ethics Committee of University of California, San Francisco, and University of Minnesota in view of the retrospective nature of the study, and all the procedures being performed were part of the routine care.
Consent for publication
Not applicable.
Conflict of interest
Ali Salavati, Mehmet Gencturk, Yasemin Koksel, Allyssa N. Schick, Steven P. Rowe, Courtney Lawhn-Heath, Jerry W. Froelich have no conflicts of interest to declare that are relevant to the content of this article. Thomas A. Hope served as a consultant toCurium and ITM and received research funding from Clovis and Philips. Thomas A. Hope also served on scientific advisory boards for Blue Earth, Ipsen. Peter R. Carroll served on an advisory board for Progenics. Felix Y. Feng has served as a consultant to Astellas, Bayer, Blue Earth, BMS, Exact Sciences, Foundation Medicine, Janssen, Myovant, Roivant, and Varian. Felix Y. Feng also serves on the scientifc advisoy boards of SerImmune and BlueStar Genomics.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Oncology - Genitourinary
Rights and permissions
About this article
Cite this article
Salavati, A., Gencturk, M., Koksel, Y. et al. A bicentric retrospective analysis of clinical utility of 18F-fluciclovine PET in biochemically recurrent prostate cancer following primary radiation therapy: is it helpful in patients with a PSA rise less than the Phoenix criteria?. Eur J Nucl Med Mol Imaging 48, 4463–4471 (2021). https://doi.org/10.1007/s00259-021-05415-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-021-05415-y