Abstract
Purpose
The aim of our study was to investigate the correlation between cfDNA concentration and fragment size fraction with FDG PET/CT- and CT-derived parameters in untreated NSCLC patient.
Methods
Fifty-three patients diagnosed of locally advanced or metastatic NSCLC who had undergone FDG PET/CT, CT and cfDNA analysis prior to any treatment were included in this retrospective study. CfDNA concentration was measured by fluorometry and fragment size fractions were determined by microchip electrophoresis. [18F]F-FDG PET/CT was performed and standardised uptake values (SUV), metabolic tumour volume (MTV) and total lesion glycolysis (TLG) were calculated for primary, extrapulmonary and total disease. CT scans were evaluated according to RECIST 1.1 criteria.
Results
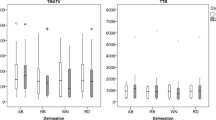
CfDNA concentration showed a positive correlation with extrapulmonary MTV (r2 = 0.36, P = 0.009), and extrapulmonary TLG (r2 = 0.35, P = 0.009) and their whole-body (wb) ratios. Higher concentrations of total cfDNA were found in patients with liver lesions. Short fragments of cfDNA (100–250 bp) showed a positive correlation with extrapulmonary MTV (r2 = 0.49, P = 0.0005) and extrapulmonary TLG (r2 = 0.39, P = 0.006) and their respective wb ratios, and a negative correlation with SUVmean (r2 = −0.31, P = 0.03) and SUVmean/SUVmax ratio (r2 = −0.34, P = 0.02). A higher fraction of short cfDNA fragments was found in patients with liver and pleural lesions.
Conclusions
This study supports the hypothesis that cfDNA concentration and short cfDNA fragment size fraction reflect the tumour burden as well as metabolic activity in advanced NSCLC patients. This suggests their suitability as complementary tests for a more accurate diagnosis of tumour metabolic behaviour and to allow personalised therapies.



Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Inamura K. Lung cancer: understanding its molecular pathology and the 2015 WHO classification. Front Oncol. 2017;7:1–7.
Pakkala S, Ramalingam SS. Personalized therapy for lung cancer: striking a moving target. JCI insight. 2018;3.
Diaz LA, Bardelli A. Liquid biopsies: genotyping circulating tumor DNA. J Clin Oncol. 2014;32:579–86.
Wiener RS, Schwartz LM, Woloshin S, Welch HG. Population-based risk for complications after transthoracic needle lung biopsy of a pulmonary nodule: an analysis of discharge records. Ann Intern Med. 2011;155:137–44.
Valpione S, Gremel G, Mundra P, Middlehurst P, Galvani E, Girotti MR, et al. Plasma total cell-free DNA (cfDNA) is a surrogate biomarker for tumour burden and a prognostic biomarker for survival in metastatic melanoma patients. Eur J Cancer. 2018;88:1–9.
Tissot C, Toffart A-C, Villar S, Souquet P-J, Merle P, Moro-Sibilot D, et al. Circulating free DNA concentration is an independent prognostic biomarker in lung cancer. Eur Respir J. 2015;46:1773–80.
Spindler K-LG, Boysen AK, Pallisgård N, Johansen JS, Tabernero J, Sørensen MM, et al. Cell-free DNA in metastatic colorectal cancer: a systematic review and meta-analysis. Oncologist. 2017;22:1049–55.
Dawson S-J, Tsui DWY, Murtaza M, Biggs H, Rueda OM, Chin S-F, et al. Analysis of circulating tumor DNA to monitor metastatic breast cancer. N Engl J Med. 2013;368:1199–209.
Singh N, Gupta S, Pandey RM, Chauhan SS, Saraya A. High levels of cell-free circulating nucleic acids in pancreatic cancer are associated with vascular encasement. Metastasis Poor Surviv Cancer Invest. 2015;33:78–85.
Winther-Larsen A, Fledelius J, Demuth C, Bylov CM, Meldgaard P, Sorensen BS. Early change in FDG-PET signal and plasma cell-free DNA level predicts erlotinib response in EGFR wild-type NSCLC patients. Transl Oncol. 2016;9:505–11.
Szpechcinski A, Chorostowska-Wynimko J, Struniawski R, Kupis W, Rudzinski P, Langfort R, et al. Cell-free DNA levels in plasma of patients with non-small-cell lung cancer and inflammatory lung disease. Br J Cancer. 2015;113:476–83.
Weng J-L, Atyah M, Zhou C-H, Ren N. Progress in quantitative technique of circulating cell free DNA and its role in cancer diagnosis and prognosis. Cancer Gene Ther. 2019;239:75–84.
Bronkhorst AJ, Ungerer V, Holdenrieder S. The emerging role of cell-free DNA as a molecular marker for cancer management. Biomol Detect Quantif. 2019;17:100087.
Underhill HR, Kitzman JO, Hellwig S, Welker NC, Daza R, Baker DN, et al. Fragment length of circulating tumor DNA. PLoS Genet. 2016;12:1–24.
Jiang P, Chan CWM, Chan KCA, Cheng SH, Wong J, Wong VWS, et al. Lengthening and shortening of plasma DNA in hepatocellular carcinoma patients. Proc Natl Acad Sci U S A. 2015;112:E1317–25.
Mouliere F, Robert B, Peyrotte E, Del Rio M, Ychou M, Molina F, et al. High fragmentation characterizes tumour-derived circulating DNA. PLoS One. 2011;6(9):e23418.
Yamamoto Y, Uemura M, Nakano K, Hayashi Y, Wang C, Ishizuya Y, et al. Increased level and fragmentation of plasma circulating cell-free DNA are diagnostic and prognostic markers for renal cell carcinoma. Oncotarget. 2018;9:20467–75.
Lapin M, Oltedal S, Tjensvoll K, Buhl T, Smaaland R, Garresori H, et al. Fragment size and level of cell-free DNA provide prognostic information in patients with advanced pancreatic cancer. J Transl Med. 2018;16:1–10.
Soliman SES, Alhanafy AM, Habib MSE, Hagag M, Ibrahem RAL. Serum circulating cell free DNA as potential diagnostic and prognostic biomarker in non small cell lung cancer. Biochem Biophys Reports. 2018;15:45–51.
Lin J, Xie G, Liao G, Wang B, Yan M, Li H, et al. Prognostic value of 18F-FDG-PET/CT in patients with nasopharyngeal carcinoma: a systematic review and meta-analysis. Oncotarget. 2017;8:33884–96.
Infante JR, Cabrera J, Rayo JI, Cruz C, Serrano J, Moreno A, et al. 18F-FDG PET/CT quantitative parameters as prognostic factor in localized and inoperable lung cancer. Rev Esp Med Nucl Imagen Mol. 2020;39:353–9.
Zhu D, Wang L, Zhang H, Chen J, Wang Y, Byanju S, et al. Prognostic value of 18F-FDG-PET/CT parameters in patients with pancreatic carcinoma: a systematic review and meta-analysis. Medicine. 2017;96:e7813.
McEvoy AC, Warburton L, Al-Ogaili Z, Celliers L, Calapre L, Pereira MR, et al. Correlation between circulating tumour DNA and metabolic tumour burden in metastatic melanoma patients. BMC Cancer. 2018;18:1–8.
Woff E, Kehagias P, Vandeputte C, Ameye L, Guiot T, Paesmans M, et al. Combining 18F-FDG PET/CT-based metabolically active tumor volume and circulating cell-free DNA significantly improves outcome prediction in chemorefractory metastatic colorectal cancer. J Nucl Med. 2019;60:1366–72.
Vizza E, Corrado G, De Angeli M, Carosi M, Mancini E, Baiocco E, et al. Serum DNA integrity index as a potential molecular biomarker in endometrial cancer. J Exp Clin Cancer Res. 2018;37:16.
Çelik F, Tan YZ, Özdemir S, Sılan F. Comparison of SUVmax values obtained from F-18 FDG PET/CT and cell-free DNA levels measured from plasma in oncology patients. Mol Imaging Radionucl Ther. 2019;28:46–52.
Winther-Larsen A, Demuth C, Fledelius J, Madsen AT, Hjorthaug K, Meldgaard P, et al. Correlation between circulating mutant DNA and metabolic tumour burden in advanced non-small cell lung cancer patients. Br J Cancer. 2017;117:704–9.
Morbelli S, Alama A, Ferrarazzo G, Coco S, Genova C, Rijavec E, et al. Circulating tumor DNA reflects tumor metabolism rather than tumor burden in chemotherapy-naive patients with advanced non–small cell lung cancer:18F-FDG PET/CT study. J Nucl Med. 2017;58:1764–9.
Nygaard AD, Holdgaard PC, Spindler KLG, Pallisgaard N, Jakobsen A. The correlation between cell-free DNA and tumour burden was estimated by PET/CT in patients with advanced NSCLC. Br J Cancer. 2014;110:363–8.
Hu Z, Chen H, Long Y, Li P, Gu Y. The main sources of circulating cell-free DNA: apoptosis, necrosis and active secretion. Crit Rev Oncol Hematol. 2021;157:103166.
Giroux DJ, Rami-Porta R, Chansky K, Crowley JJ, Groome PA, Postmus PE, et al. The IASLC Lung Cancer Staging Project: data elements for the prospective project. J Thorac Oncol. 2009;4:679–83.
Vesselle H, Salskov A, Turcotte E, Wiens L, Schmidt R, Jordan CD, et al. Relationship between non-small cell lung cancer FDG uptake at PET, tumor histology, and Ki-67 proliferation index. J Thorac Oncol. 2008;3:971–8.
Cappabianca S, Porto A, Petrillo M, Greco B, Reginelli A, Ronza F, et al. Preliminary study on the correlation between grading and histology of solitary pulmonary nodules and contrast enhancement and [18F]fluorodeoxyglucose standardised uptake value after evaluation by dynamic multiphase CT and PET/CT. J Clin Pathol. 2011;64:114–9.
Chiu C-H, Yeh Y-C, Lin K-H, Wu Y-C, Lee Y-C, Chou T-Y, et al. Histological subtypes of lung adenocarcinoma have differential 18F-fluorodeoxyglucose uptakes on the positron emission tomography/computed tomography scan. J Thorac Oncol. 2011;6:1697–703.
Stewart CM, Tsui DWY. Circulating cell-free DNA for non-invasive cancer management. Cancer Gene Ther. 2018;228–229:169–79.
Sorber L, Zwaenepoel K, Jacobs J, De Winne K, Goethals S, Reclusa P, et al. Circulating cell-free DNA and RNA analysis as liquid biopsy: optimal centrifugation protocol. Cancers (Basel). 2019;11:458.
Bronkhorst AJ, Aucamp J, Pretorius PJ. Cell-free DNA: Preanalytical variables. Clin Chim Acta. 2015;450:243–53.
Shi J, Zhang R, Li J, Zhang R. Size profile of cell-free DNA: a beacon guiding the practice and innovation of clinical testing. Theranostics. 2020;10:4737–48.
Dooms C, van Baardwijk A, Verbeken E, van Suylen RJ, Stroobants S, De Ruysscher D, et al. Association between 18F-fluoro-2-deoxy-D-glucose uptake values and tumor vitality: prognostic value of positron emission tomography in early-stage non-small cell lung cancer. J Thorac Oncol. 2009;4:822–8.
Wang R-A, Li Q-L, Li Z-S, Zheng P-J, Zhang H-Z, Huang X-F, et al. Apoptosis drives cancer cells proliferate and metastasize. J Cell Mol Med. 2013;17:205–11.
Jahr S, Hentze H, Englisch S, Hardt D, Fackelmayer FO, Hesch R-D, et al. DNA fragments in the blood plasma of cancer patients: quantitations and evidence for their origin from apoptotic and necrotic cells. Cancer Res. 2001;61:1659–65.
Kaira K, Okumura T, Ohde Y, Takahashi T, Murakami H, Oriuchi N, et al. Correlation between 18F-FDG uptake on PET and molecular biology in metastatic pulmonary tumors. J Nucl Med. 2011;52:705–11.
Acknowledgements
The authors would like to dedicate this manuscript to Dr. Rafael Molina, who recently passed away. His dedication and passion to the field of cancer diagnosis were an inspiration to all of us. The authors thank the patients for their participation in the study and the study staff who were involved in collecting specimen material at the study sites. The authors would like to express our gratitude to Dr. James Hugall for providing language assistance for the manuscript.
Funding
Hoffman-La Roche Ltd. supplied the reagent cobas® cfDNA Sample Preparation Kit for cfDNA extraction.
Author information
Authors and Affiliations
Contributions
Conceptualisation: JM. González de Aledo-Castillo, I. Vollmer, P. Paredes, JA. Puig-Butillé; data curation: JM. González de Aledo-Castillo, S. Casanueva-Eliceiry, A. Soler, V. Pastor, N. Reguart, N. Viñolas, R. Reyes, I. Vollmer, P. Paredes, JA. Puig-Butillé; formal analysis: JM. González de Aledo-Castillo; investigation: JM. González de Aledo-Castillo, I. Vollmer, P. Paredes, JA. Puig-Butillé; methodology: JM. González de Aledo-Castillo, JA. Puig-Butillé; project administration: P. Paredes, JA. Puig-Butillé; resources: P. Paredes, I. Vollmer, D. Fuster, N. Reguart, JA. Puig-Butillé; supervision: P. Paredes, JA. Puig-Butillé; visualisation: JM. González de Aledo-Castillo, I. Vollmer, P. Paredes, JA. Puig-Butillé; writing – original draft: JM. González de Aledo-Castillo: writing – review and editing: JM. González de Aledo-Castillo, I. Vollmer, P. Paredes, N. Reguart, JA. Puig-Butillé. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Hospital Clinic de Barcelona (approval registration number HCB/2016/0889).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Oncology - Chest.
Rights and permissions
About this article
Cite this article
González de Aledo-Castillo, J., Casanueva-Eliceiry, S., Soler-Perromat, A. et al. Cell-free DNA concentration and fragment size fraction correlate with FDG PET/CT-derived parameters in NSCLC patients. Eur J Nucl Med Mol Imaging 48, 3631–3642 (2021). https://doi.org/10.1007/s00259-021-05306-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-021-05306-2