Abstract
Purpose
Up to 25% of patients diagnosed as idiopathic Parkinson’s disease (IPD) have an atypical parkinsonian syndrome (APS). We had previously validated an automated image-based algorithm to discriminate between IPD, multiple system atrophy (MSA), and progressive supranuclear palsy (PSP). While the algorithm was accurate with respect to the final clinical diagnosis after long-term expert follow-up, its relationship to the initial referral diagnosis and to the neuropathological gold standard is not known.
Methods
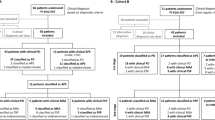
Patients with an uncertain diagnosis of parkinsonism were referred for 18F-fluorodeoxyglucose (FDG) PET to classify patients as IPD or as APS based on the automated algorithm. Patients were followed by a movement disorder specialist and subsequently underwent neuropathological examination. The image-based classification was compared to the neuropathological diagnosis in 15 patients with parkinsonism.
Results
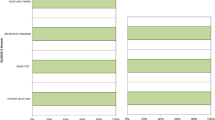
At the time of referral to PET, the clinical impression was only 66.7% accurate. The algorithm correctly identified 80% of the cases as IPD or APS (p = 0.02) and 87.5% of the APS cases as MSA or PSP (p = 0.03). The final clinical diagnosis was 93.3% accurate (p < 0.001), but needed several years of expert follow-up.
Conclusion
The image-based classifications agreed well with autopsy and can help to improve diagnostic accuracy during the period of clinical uncertainty.




Similar content being viewed by others
References
Rizzo G, Copetti M, Arcuti S, Martino D, Fontana A, Logroscino G. Accuracy of clinical diagnosis of Parkinson disease. Neurology. 2016;86:566–76.
Fahn S, Jankovic J. Principles and practice of movement disorders. Philadelphia: Churchill Livingstone Elsevier; 2007.
Krismer F, Wenning GK. Multiple system atrophy: insights into a rare and debilitating movement disorder. Nat Rev Neurol. 2017;13:232–43.
Obeso JA, Stamelou M, Goetz CG, Poewe W, Lang AE, Weintraub D, et al. Past, present, and future of Parkinson’s disease: a special essay on the 200th Anniversary of the Shaking Palsy. Mov Disord. 2017;32:1264–310.
Rus T, Tomše P, Jensterle L, Ležaić L, Stokin CL, Popović M, et al. Atypical clinical presentation of pathologically proven Parkinson’s disease: the role of Parkinson’s disease related metabolic pattern. Park Relat Disord. 2020;78:1–3.
Adler CH, Beach TG, Hentz JG, Shill HA, Caviness JN, Driver-Dunckley E, et al. Low clinical diagnostic accuracy of early vs advanced Parkinson disease: clinicopathologic study. Neurology. 2014;83:406–12.
Meyer PT, Frings L, Rücker G, Hellwig S. 18F-FDG PET in parkinsonism: differential diagnosis and evaluation of cognitive impairment. J Nucl Med. 2017;58:1888–98.
Meles SK, Teune LK, de Jong BM, Dierckx RA, Leenders KL. Metabolic imaging in Parkinson disease. J Nucl Med. 2017;58:23–8.
Strafella AP, Bohnen NI, Perlmutter JS, Eidelberg D, Pavese N, Van Eimeren T, et al. Molecular imaging to track Parkinson’s disease and atypical parkinsonisms: new imaging frontiers. Mov Disord. 2017;32:181–92.
Caminiti SP, Alongi P, Majno L, Volontè MA, Cerami C, Gianolli L, et al. Evaluation of an optimized [18F]fluoro-deoxy-glucose positron emission tomography voxel-wise method to early support differential diagnosis in atypical Parkinsonian disorders. Eur J Neurol. 2017;24:687–e26.
Antonini A, Kazumata K, Feigin A, Mandel F, Dhawan V, Margouleff C, et al. Differential diagnosis of parkinsonism with [ 18F ] fluorodeoxyglucose and PET. 1998;13:268–74.
Tang CC, Poston KL, Eckert T, Feigin A, Frucht S, Gudesblatt M, et al. Differential diagnosis of parkinsonism: a metabolic imaging study using pattern analysis. Lancet Neurol. 2010;9:149–58.
Schindlbeck KA, Eidelberg D. Network imaging biomarkers: insights and clinical applications in Parkinson’s disease. Lancet Neurol. 2018;17:629–40.
Tripathi M, Tang CC, Feigin A, De Lucia I, Nazem A, Dhawan V, et al. Automated differential diagnosis of early parkinsonism using metabolic brain networks: a validation study. J Nucl Med. 2016;57:60–6.
Rus T, Tomše P, Jensterle L, Grmek M, Pirtošek Z, Eidelberg D, et al. Differential diagnosis of parkinsonian syndromes: a comparison of clinical and automated - metabolic brain patterns’ based approach. Eur J Nucl Med Mol Imaging. 2020;47:2901–10.
Markaki I, Tang CC, Lilja Lindström M, Eidelberg D, Svenningsson P, Savitcheva I. Automated metabolic pattern analysis in the differential diagnosis of parkinsonism in a Swedish cohort [abstract]. Mov Disord. 2019;34.
Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–4.
Gilman S, Wenning GK, Low PA, Brooks DJ, Mathias CJ, Trojanowski JQ, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008;71:670–6.
Hauw JJ, Daniel SE, Dickson D, Horoupian DS, Jellinger K, Lantos PL, et al. Preliminary NINDS neuropathologic criteria for Steele-Richardson-Olszewski syndrome (progressive supranuclear palsy). Neurology. 1994;44:2015–9.
Ma Y, Tang C, Spetsieris PG, Dhawan V, Eidelberg D. Abnormal metabolic network activity in Parkinson’s disease: test-retest reproducibility. J Cereb Blood Flow Metab. 2007;27:597–605.
Spetsieris PG, Eidelberg D. Scaled subprofile modeling of resting state imaging data in Parkinson’s disease: methodological issues. Neuroimage. 2011;54:2899–914.
Papp MI, Lantos PL. The distribution of oligodendroglial inclusions in multiple system atrophy and its relevance to clinical symptomatology. Brain. 1994;117:235–43.
Litvan I, Hauw JJ, Bartko JJ, Lantos PL, Daniel SE, Horoupian DS, et al. Validity and reliability of the preliminary NINDS neuropathologic criteria for progressive supranuclear palsy and related disorders. J Neuropathol Exp Neurol. 1996;55:97–105.
Dickson DW, Lin WL, Liu WK, Yen SH. Multiple system atrophy: a sporadic synucleinopathy. Brain Pathol. 1999;9:721–32.
Vonsattel JPG, del Amaya MP, Keller CE. Twenty-first century brain banking. Processing brains for research: the Columbia University methods. Acta Neuropathol. 2008;115:509–32.
Hughes AJ, Daniel SE, Ben-Shlomo Y, Lees AJ. The accuracy of diagnosis of parkinsonian syndromes in a specialist movement disorder service. Brain. 2002;125:861–70.
Poston KL, Tang CC, Eckert T, Dhawan V, Frucht S, Vonsattel JP, et al. Network correlates of disease severity in multiple system atrophy. Neurology. 2012;78:1237–44.
Habeck C, Foster NL, Perneczky R, Kurz A, Alexopoulos P, Koeppe RA, et al. Multivariate and univariate neuroimaging biomarkers of Alzheimer’s disease. Neuroimage. 2008;40:1503–15.
Teune LK, Renken RJ, Mudali D, De Jong BM, Dierckx RA, Roerdink JBTM, et al. Validation of parkinsonian disease-related metabolic brain patterns. Mov Disord. 2013;28:547–51.
Mattis PJ, Niethammer M, Sako W, Tang CC, Nazem A, Gordon ML, et al. Distinct brain networks underlie cognitive dysfunction in Parkinson and Alzheimer diseases. Neurology. 2016;87:1925–33.
Jecmenica Lukic M, Kurz C, Respondek G, Grau-Rivera O, Compta Y, Gelpi E, et al. Copathology in progressive supranuclear palsy: does it matter? Mov Disord. 2020;35:984–93.
Giagkou N, Stamelou M. Emerging drugs for progressive supranuclear palsy. Expert Opin Emerg Drugs. 2019;24:83–92.
Woo C-W, Chang LJ, Lindquist MA, Wager TD. Building better biomarkers: brain models in translational neuroimaging. Nat Neurosci. 2017;20:365–77.
Niethammer M, Tang CC, Vo A, Nguyen N, Spetsieris P, Dhawan V, et al. Gene therapy reduces Parkinson’s disease symptoms by reorganizing functional brain connectivity. Sci Transl Med. United States. 2018;10:aau0713.
Acknowledgements
The authors thank Vicky Brandt and Dr. Martin Niethammer for helpful discussions and suggestions. We also thank Rosie Persaud for her invaluable assistance with coordinating data collection.
Availability of data and material
Deidentified data will be made available on reasonable request from interested investigators for the purpose of replicating results.
Funding
Aspects of this work were supported by the National Institute of Neurological Disorders and Stroke (P50 NS 071675 (Morris K. Udall Center of Excellence for Parkinson’s Disease Research at The Feinstein Institute for Medical Research) to D.E.). K.A.S. is supported by the Leopoldina Fellowship Program of the German National Academy of Sciences Leopoldina (LDS 2016-08) and the Postdoctoral Fellowship Grant No. PF-FBS-1929 from the Parkinson’s Foundation.
Author information
Authors and Affiliations
Contributions
K.A.S., D.K.G., J.-P.V., S.F., and D.E. contributed to the conception and design of the study; K.A.S., D.K.G., C.C.T., S.A.O., K.L.P., Y.Y.C., V.D., J.-P.V., S.F., and D.E. contributed to the acquisition and analysis of the data; and K.A.S. and D.E. drafted the manuscript and prepared the figures; D.K.G., C.C.T., S.A.O., K.L.P., Y.Y.C., V.D., J.-P.V., and S.F. reviewed the manuscript for intellectual content.
Corresponding author
Ethics declarations
Ethics approval
Ethical permission for this study was obtained from the Institutional Review Board of the participating institutions (Columbia University and Northwell Health).
Consent to participate
A waiver of consent was granted for this study.
Conflict of interest
K.L.P. has received clinical trial grants from Sanofi and AstraZeneca (unrelated to manuscript) and consulting fees from Allergan and CuraSen (unrelated to manuscript). D.E. serves on the scientific advisory boards of and has received fees from The Michael J. Fox Foundation for Parkinson’s Research and Ovid Therapeutics (unrelated to manuscript); receives consulting fees from MeiraGTx (unrelated to manuscript); has received grants from NIH (NINDS, NIAID) (unrelated to manuscript); and is the coinventor of patents re: Markers for use in screening patients for nervous system dysfunction and a method and apparatus for using same, without financial gain. All other authors disclose no relevant conflict of interest.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the National Institute of Neurological Disorders and Stroke.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Neurology – Movement disorders.
Supplementary information
ESM 1
(DOCX 31 kb)
Rights and permissions
About this article
Cite this article
Schindlbeck, K.A., Gupta, D.K., Tang, C.C. et al. Neuropathological correlation supports automated image-based differential diagnosis in parkinsonism. Eur J Nucl Med Mol Imaging 48, 3522–3529 (2021). https://doi.org/10.1007/s00259-021-05302-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-021-05302-6