Abstract
Introduction
Volume changes induced by selective internal radiation therapy (SIRT) may increase the possibility of tumor resection in patients with insufficient future liver remnant (FLR). The aim was to identify dosimetric and clinical parameters associated with contralateral hepatic hypertrophy after lobar/extended lobar SIRT with 90Y-resin microspheres.
Materials and methods
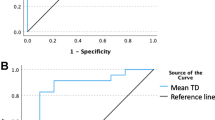
Patients underwent 90Y PET/CT after lobar or extended lobar (right + segment IV) SIRT. 90Y voxel dosimetry was retrospectively performed (PLANET Dose; DOSIsoft SA). Mean absorbed doses to tumoral/non-tumoral-treated volumes (NTL) and dose-volume histograms were extracted. Clinical variables were collected. Patients were stratified by FLR at baseline (T0-FLR): < 30% (would require hypertrophy) and ≥ 30%. Changes in volume of the treated, non-treated liver, and FLR were calculated at < 2 (T1), 2–5 (T2), and 6–12 months (T3) post-SIRT. Univariable and multivariable regression analyses were performed to identify predictors of atrophy, hypertrophy, and increase in FLR. The best cut-off value to predict an increase of FLR to ≥ 40% was defined using ROC analysis.
Results
Fifty-six patients were studied; most had primary liver tumors (71.4%), 40.4% had cirrhosis, and 39.3% had been previously treated with chemotherapy. FLR in patients with T0-FLR < 30% increased progressively (T0: 25.2%; T1: 32.7%; T2: 38.1%; T3: 44.7%). No dosimetric parameter predicted atrophy. Both NTL-Dmean and NTL-V30 (fraction of NTL exposed to ≥ 30 Gy) were predictive of increase in FLR in patients with T0 FLR < 30%, the latter also in the total cohort of patients. Hypertrophy was not significantly associated with tumor dose or tumor size. When ≥ 49% of NTL received ≥ 30 Gy, FLR increased to ≥ 40% (accuracy: 76.4% in all patients and 80.95% in T0-FLR < 30% patients).
Conclusion
NTL-Dmean and NTL exposed to ≥ 30 Gy (NTL-V30) were most significantly associated with increase in FLR (particularly among patients with T0-FLR < 30%). When half of NTL received ≥ 30 Gy, FLR increased to ≥ 40%, with higher accuracy among patients with T0-FLR < 30%.



Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Braat AJAT, Smits MLJ, Braat MNGJA, van den Hoven AF, Prince JF, de Jong HWAM, et al. 90Y hepatic radioembolization: an update on current practice and recent developments. J Nucl Med. 2015;56:1079–87.
Sangro B, Gil-Alzugaray B, Rodriguez J, Sola I, Martinez-Cuesta A, Viudez A, et al. Liver disease induced by radioembolization of liver tumors: description and possible risk factors. Cancer. 2008;112:1538–46.
Seidensticker M, Burak M, Kalinski T, Garlipp B, Koelble K, Wust P, et al. Radiation-induced liver damage: correlation of histopathology with hepatobiliary magnetic resonance imaging, a feasibility study. Cardiovasc Intervent Radiol. 2015;38:213–21.
Kim RD, Kim JS, Watanabe G, Mohuczy D, Behrns KE. Liver regeneration and the atrophy-hypertrophy complex. Semin Intervent Radiol Thieme Medical Publishers. 2008;25:92–103.
Jakobs TF, Saleem S, Atassi B, Reda E, Lewandowski RJ, Yaghmai V, et al. Fibrosis, portal hypertension, and hepatic volume changes induced by intra-arterial radiotherapy with90Yttrium microspheres. Dig Dis Sci. 2008;53:2556–63.
Gaba RC, Lewandowski RJ, Kulik LM, Riaz A, Ibrahim SM, Mulcahy MF, et al. Radiation lobectomy: preliminary findings of hepatic volumetric response to lobar yttrium-90 radioembolization. Ann Surg Oncol. 2009;16:1587–96.
Vouche M, Lewandowski RJ, Atassi R, Memon K, Gates VL, Ryu RK, et al. Radiation lobectomy: time-dependent analysis of future liver remnant volume in unresectable liver cancer as a bridge to resection. J Hepatol European Association for the Study of the Liver. 2013;59:1029–36.
Edeline J, Lenoir L, Boudjema K, Rolland Y, Boulic A, Le Du F, et al. Volumetric changes after 90Y radioembolization for hepatocellular carcinoma in cirrhosis: an option to portal vein embolization in a preoperative setting? Ann Surg Oncol. 2013;20:2518–25.
Ahmadzadehfar H, Meyer C, Ezziddin S, Sabet A, Hoff-Meyer A, Muckle M, et al. Hepatic volume changes induced by radioembolization with 90Y resin microspheres. A single-centre study. Eur J Nucl Med Mol Imaging. 2013;40:80–90.
Garlipp B, de Baere T, Damm R, Irmscher R, van Buskirk M, Stübs P, et al. Left-liver hypertrophy after therapeutic right-liver radioembolization is substantial but less than after portal vein embolization. Hepatology John Wiley and Sons Ltd. 2014;59:1864–73.
Teo JY, Goh BKP, Cheah FK, Allen JC, Lo RHG, Ng DCE, et al. Underlying liver disease influences volumetric changes in the spared hemiliver after selective internal radiation therapy with 90Y in patients with hepatocellular carcinoma. J Dig Dis Blackwell Publishing. 2014;15:444–50.
Teo JY, Allen JC, Ng DC, Choo SP, Tai DWM, Chang JPE, et al. A systematic review of contralateral liver lobe hypertrophy after unilobar selective internal radiation therapy with Y90. Hpb. International Hepato-Pancreato-Biliary Association Inc. 2016;18:7–12.
Fernández-Ros N, Silva N, Bilbao JI, Iñarrairaegui M, Benito A, D’Avola D, et al. Partial liver volume radioembolization induces hypertrophy in the spared hemiliver and no major signs of portal hypertension. HPB. 2014;16:243–9.
Goh BKP. Measured versus estimated total liver volume to preoperatively assess the adequacy of future liver remnant. Ann Surg. 2015;262:e72.
Tanaka K, Shimada H, Matsuo K, Ueda M, Endo I, Togo S. Remnant liver regeneration after two-stage hepatectomy for multiple bilobar colorectal metastases. Eur J Surg Oncol. 2007;33:329–35.
Hemming AW, Reed AI, Howard RJ, Fujita S, Hochwald SN, Caridi JG, et al. Preoperative portal vein embolization for extended hepatectomy. Ann Surg. 2003;237:686–91discussion 691-3.
Lassmann M, Eberlein U. The relevance of dosimetry in precision medicine. J Nucl Med. 2018;59:1494–9.
Chiesa C, Maccauro M, Romito R, Spreafico C, Pellizzari S, Negri A, et al. Need, feasibility and convenience of dosimetric treatment planning in liver selective internal radiation therapy with (90)Y microspheres: the experience of the National Tumor Institute of Milan. Q J Nucl Med Mol Imaging. 2011;55:168–97.
Lam MGEH, Goris ML, Iagaru AH, Mittra ES, Louie JD, Sze DY. Prognostic utility of 90Y radioembolization dosimetry based on fusion 99mTc-macroaggregated albumin-99mTc-sulfur colloid SPECT. J Nucl Med. 2013;54:2055–61.
Tong AKT, Kao YH, Too C, Chin KFW, Ng DCE, Chow PKH. Yttrium-90 hepatic radioembolization: clinical review and current techniques in interventional radiology and personalized dosimetry. Br J Radiol. 2016;89.
Garin E, Lenoir L, Rolland Y, Edeline J, Mesbah H, Laffont S, et al. Dosimetry based on 99mTc-macroaggregated albumin SPECT/CT accurately predicts tumor response and survival in hepatocellular carcinoma patients treated with 90Y-loaded glass microspheres: preliminary results. J Nucl Med. 2012;53:255–63.
Garin E, Rolland Y, Laffont S, Edeline J. Clinical impact of 99mTc-MAA SPECT/CT-based dosimetry in the radioembolization of liver malignancies with 90Y-loaded microspheres. Eur J Nucl Med Mol Imaging. 2016;43:559–75.
Palard X, Edeline J, Rolland Y, Le Sourd S, Pracht M, Laffont S, et al. Dosimetric parameters predicting contralateral liver hypertrophy after unilobar radioembolization of hepatocellular carcinoma. Eur J Nucl Med Mol Imaging. 2018;45:392–401.
Gil-Alzugaray B, Chopitea A, Iñarrairaegui M, Bilbao JI, Rodriguez-Fraile M, Rodriguez J, et al. Prognostic factors and prevention of radioembolization-induced liver disease. Hepatology Wiley-Blackwell. 2013;57:1078–87.
Sirtex Medical. SIR-Spheres® Y-90 resin microspheres activity chart [Internet]. 2020 [cited 2020 Oct 2]. Available from: https://www.sirtex.com/media/168731/activity-chart-apm-us-368-v1-0220.pdf
Martí-Climent JM, Prieto E, Elosúa C, Rodríguez-Fraile M, Domínguez-Prado I, Vigil C, et al. PET optimization for improved assessment and accurate quantification of 90Y-microsphere biodistribution after radioembolization. Med Phys. John Wiley and Sons Ltd. 2014;41:092503.
Peterson JL, Vallow LA, Johnson DW, Heckman MG, Diehl NN, Smith AA, et al. Complications after 90Y microsphere radioembolization for unresectable hepatic tumors: an evaluation of 112 patients. Brachytherapy Elsevier. 2013;12:573–9.
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–16.
Guglielmi A, Ruzzenente A, Conci S, Valdegamberi A, Iacono C. How much remnant is enough in liver resection? Dig Surg. 2012;29:6–17.
Goebel J, Sulke M, Lazik-Palm A, Goebel T, Dechêne A, Bellendorf A, et al. Factors associated with contralateral liver hypertrophy after unilateral radioembolization for hepatocellular carcinoma. PLoS One. 2017;12:e0181488.
Alsultan AA, Braat AJAT, Smits MLJ, Barentsz MW, Bastiaannet R, Bruijnen RCG, et al. Current status and future direction of hepatic radioembolisation. Clin Oncol. 2020;33:106–16.
Pasciak AS, Bourgeois AC, Bradley YC. A microdosimetric analysis of absorbed dose to tumor as a function of number of microspheres per unit volume in 90Y Radioembolization. J Nucl Med. 2016;57:1020–6.
van Roekel C, Reinders MTM, van der Velden S, Lam MGEH, Braat MNGJA. Hepatobiliary imaging in liver-directed treatments. Semin Nucl Med. Elsevier Inc.; 2019;49:227–36.
Braat MNGJA, de Jong HW, Seinstra BA, Scholten M V., van den Bosch MAAJ, Lam MGEH. Hepatobiliary scintigraphy may improve radioembolization treatment planning in HCC patients. EJNMMI Res. EJNMMI Research; 2017;7.
Bilbao JI, De Martino A, De Luis E, Díaz-Dorronsoro L, Alonso-Burgos A, Martínez De La Cuesta A, et al. Biocompatibility, inflammatory response, and recannalization characteristics of nonradioactive resin microspheres: histological findings. Cardiovasc Intervent Radiol. 2009;32:727–36.
Liu H, Zhu S. Present status and future perspectives of preoperative portal vein embolization. Am J Surg Elsevier. 2009;197:686–90.
Acknowledgements
The authors gratefully acknowledge the effort and the excellent technical support of the Cyclotron and PET/CT staff.
Code availability
PET-based voxel dosimetry was performed using a dedicated treatment planning system (PLANET Dose; DOSIsoft SA).
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
The institutional ethics committee approved the protocol (212/2019) for this retrospective study. Informed consent was waived.
Consent to participate
NA.
Consent for publication
NA.
Conflict of interest
M.R.-F. and M.I. are paid speakers for Sirtex Medical Europe GmbH. B.S. is a paid consultant and speaker for Sirtex Medical and BTG. The authors declare no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Dosimetry
Supplementary information
ESM 1
(DOCX 22 kb)
Rights and permissions
About this article
Cite this article
Grisanti, F., Prieto, E., Bastidas, J.F. et al. 3D voxel-based dosimetry to predict contralateral hypertrophy and an adequate future liver remnant after lobar radioembolization. Eur J Nucl Med Mol Imaging 48, 3048–3057 (2021). https://doi.org/10.1007/s00259-021-05272-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-021-05272-9