Abstract
Aim
Total thyroidectomy and risk-adapted 131-radioiodine therapy (RaIT) are the treatments of choice in differentiated thyroid cancer (DTC) patients. The response to treatments is assessed 6–12 months after RaIT. However, thyroglobulin (Tg) values obtained just before RaIT also provide reliable informations on patients’outcome. As available data were mostly obtained in hypothyroid status, we evaluated the predictive role of preablation-Tg in patients underwent RaIT after rhTSH stimulation.
Material and methods
We enrolled 299 low-to-intermediate risk DTC patients underwent rhTSH-stimulated RaIT (standard protocol). Serum Tg levels were measured before rhTSH administration (basal Tg), before RaIT (early-stimulated Tg), and 2 days after RaIT (late-stimulated Tg). The early response assessment was done 12 months after RaIT according to 2015 American Thyroid Association (2015 ATA) criteria.
Results
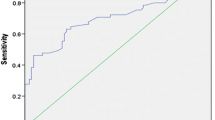
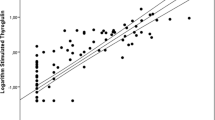
Most patients (277/299, 92.6%) had an excellent response (ER) to RaIT, while 15/299 (5.1%) and 7/299 (2.3%) patients showed biochemical incomplete/indeterminate response or persistent structural disease, respectively. At receiver operating characteristic analysis, the optimal cutoff to predict ER was set at 1.55 (AUC = 0.792), 2.6 (AUC = 0.931), and 4.9 (AUC = 0.874) ng/mL, for basal, early-, and late-stimulated Tg, respectively. The overall sensitivity, specificity, accuracy, positive predictive value (PPV), and negative predictive value (NPV) for basal, early-, and late-stimulated Tg were 50%, 96.7%, 93.3%, 55%, and 96.1%; 90.9%, 84.5%, 84.9%, 31.7%, and 99.1%; and 90.9%, 71.8%, 73.2%, 20.4%, and 99%, respectively. In univariate and multivariate logistic regression analysis, early-stimulated Tg cutoff resulted as an independent prognostic marker for predicting ER regardless of gender, age, histotype, histological variant, tumor size, risk classification, and stage of disease.
Conclusion
Early-stimulated Tg is a reliable diagnostic tool for predicting the response to primary treatment of DTC.




Similar content being viewed by others
Data availability
available.
References
Lim H, Devesa SS, Sosa JA, et al. Trends in thyroid cancer incidence and mortality in the United States, 1974-2013. JAMA. 2017;317(13):1338–48.
Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973-2002. JAMA. 2006;295(18):2164–7.
Horn-Ross PL, Lichtensztajn DY, Clarke CA, et al. Continued rapid increase in thyroid cancer incidence in California: trends by patient, tumor, and neighborhood characteristics. Cancer Epidemiol Biomark Prev. 2014;23(6):1067–79.
Cancer Statistics Review, 1975–2013 National Cancer Institute. Bethesda, MD, based on November 2015 SEER data submission.
Tuttle RM, Ahuja S, Avram AM, et al. Controversies, consensus, and collaboration in the use of 131I therapy in differentiated thyroid Cancer: a joint statement from the American Thyroid Association, the European Association of Nuclear Medicine, the Society of Nuclear Medicine and Molecular Imaging, and the European Thyroid Association. Thyroid. 2019;29(4):461–70.
Giovanella L, Duntas LH. MANAGEMENT OF ENDOCRINE DISEASE: the role of rhTSH in the management of differentiated thyroid cancer: pros and cons. Eur J Endocrinol. 2019;181(4):133–45.
Campennì A, Barbaro D, Guzzo M, et al. Personalized management of differentiated thyroid cancer in real life -practical guidance from a multidisciplinary panel of experts. Endocrine. 2020. https://doi.org/10.1007/s12020-020-02418-x.
Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1–133.
Luster M, Clarke SE, Dietlein M, et al. Guidelines for radioiodine therapy of differentiated thyroid cancer. Eur J Nucl Med Mol Imaging. 2008;35(10):1941–59.
Park HJ, Jeong GC, Kwon SY, et al. Stimulated serum thyroglobulin level at the time of first dose of radioactive iodine therapy is the most predictive factor for therapeutic failure in patients with papillary thyroid carcinoma. Nucl Med Mol Imaging. 2014;48(4):255–61.
Giovanella L, Ceriani L, Ghelfo A, et al. Thyroglobulin assay 4 weeks after thyroidectomy predicts outcome in low-risk papillary thyroid carcinoma. Clin Chem Lab Med. 2005;43(8):843–7.
Kim TY, Kim WB, Kim ES, et al. Serum thyroglobulin levels at the time of 131I remnant ablation just after thyroidectomy are useful for early prediction of clinical recurrence in low-risk patients with differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2005;90(3):1440–5.
Webb RC, Howard RS, Stojadinovic A, et al. The utility of serum thyroglobulin measurement at the time of remnant ablation for predicting disease-free status in patients with differentiated thyroid cancer: a meta-analysis involving 3947 patients. J Clin Endocrinol Metab. 2012;97(8):2754–63.
Park HJ, Min JJ, Bom HS, et al. Early stimulated thyroglobulin for response prediction after recombinant human thyrotropin-aided radioiodine therapy. Ann Nucl Med. 2017;31(8):616–22.
Torres MS, Ramirez L, Simkin PH, et al. Effect of various doses of recombinant human thyrotropin on the thyroid radioactive iodine uptake and serum levels of thyroid hormones and thyroglobulin in normal subjects. J Clin Endocrinol Metab. 2001;86(4):1660–4.
Weiss R, Magner J. Serial measurements of serum thyroglobulin in response to recombinant human thyrotropin stimulation. Thyroid. 2015;25(6):708–10.
Ciappuccini R, Hardouin J, Heutte N, et al. Stimulated thyroglobulin level at ablation in differentiated thyroid cancer: the impact of treatment preparation modalities and tumor burden. Eur J Endocrinol. 2014;171(2):247–52.
Taïeb D, Lussato D, Guedj E, et al. Early sequential changes in serum thyroglobulin after radioiodine ablation for thyroid cancer: possible clinical implications for recombinant human thyrotropin-aided therapy. Thyroid. 2006;16(2):177–9.
Kim YI, Im HJ, Paeng JC, et al. Serum thyroglobulin level after radioiodine therapy (Day 3) to predict successful ablation of thyroid remnant in postoperative thyroid cancer. Ann Nucl Med. 2015;29(2):184–9.
Stevic I, Dembinski TC, Pathak KA, et al. Transient early increase in thyroglobulin levels post-radioiodine ablation in patients with differentiated thyroid cancer. Clin Biochem. 2015;48(10–11):658–61.
Melo M, Costa G, Ribeiro C, et al. Stimulated thyroglobulin at recombinant human TSH-aided ablation predicts disease-free status one year later. J Clin Endocrinol Metab. 2013;98(11):4364–72.
Campennì A, Giovanella L, Siracusa M, et al. Is malignant nodule topography an additional risk factor for metastatic disease in low-risk differentiated thyroid cancer? Thyroid. 2014;24(11):1607–11.
Campennì A, Giovanella L, Pignata SA, et al. Undetectable or low (<1 ng/ml) postsurgical thyroglobulin values do not rule out metastases in early stage differentiated thyroid cancer patients. Oncotarget. 2018;9(25):17491–500.
Amin MB, Edge S, Greene F, Byrd DR, et al. AJCC cancer staging manual. 8th ed. Switzerland: Springer International Publishing; 2017.
Giovanella L, Ceriani L, Suriano S, et al. Thyroglobulin measurement before rhTSH-aided 131I ablation in detecting metastases from differentiated thyroid carcinoma. Clin Endocrinol. 2008;69(4):659–63.
Author information
Authors and Affiliations
Contributions
Alfredo Campenni: contributed to conception, design, and wrote the manuscript.
Rosaria Maddalena Ruggeri: acquired, analyzed, and interpreted the data.
Massimiliano Siracusa: acquired, analyzed, and interpreted the data.
Alessio Danilo Comis: acquired, analyzed, and interpreted the data.
Davide Romano: acquired, analyzed and interpreted the data.
Antonio Vento: acquired, analyzed, and interpreted the data.
Helena Lanzafame: acquired, analyzed, and interpreted the data.
Francesca Capoccetti: acquired, analyzed, and interpreted the data.
Angela Alibrandi: wrote the statistical analysis.
Sergio Baldari: revised the manuscript.
Luca Giovanella: contributed to conception, design and revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was approved by the Ente Ospedaliero Cantonale Institutional Review Board and the Canton Tessin Ethical Committee, Bellinzona (Switzerland) [reference BASEC 2019–00662] and the institutional review board of the Medical Faculty of the University of Messina. All procedures performed were in accordance with the principles of the 1964 Declaration of Helsinki and its later amendments.
Informed consent
In all patients, written informed consent for both diagnostic examinations and therapy were always obtained prior to the procedures.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Endocrinology.
Rights and permissions
About this article
Cite this article
Campennì, A., Ruggeri, R.M., Siracusa, M. et al. Early preablation rhTSH-stimulated thyroglobulin predicts outcome of differentiated thyroid cancer (DTC) patients. Eur J Nucl Med Mol Imaging 48, 2466–2475 (2021). https://doi.org/10.1007/s00259-020-05153-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-020-05153-7