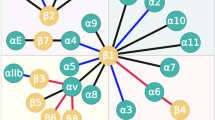
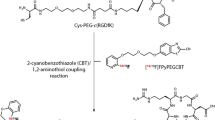
Abstract
Introduction
Non-invasive imaging techniques (especially single-photon emission tomography and positron emission tomography) apply several RGD-based imaging ligands developed during a vast number of preclinical and clinical investigations. The RGD (Arg-Gly-Asp) sequence is a binding moiety for a large selection of adhesive extracellular matrix and cell surface proteins. Since the first identification of this sequence as the shortest sequence required for recognition in fibronectin during the 1980s, fundamental research regarding the molecular mechanisms of integrin action have paved the way for development of several pharmaceuticals and radiopharmaceuticals with clinical applications. Ligands recognizing RGD may be developed for use in the monitoring of these interactions (benign or pathological). Although RGD-based molecular imaging has been actively investigated for oncological purposes, their utilization towards non-oncology applications remains relatively under-exploited.
Methods and Scope
This review highlights the new non-oncologic applications of RGD-based tracers (with the focus on single-photon emission tomography and positron emission tomography). The focus is on the last 10 years of scientific literature (2009–2020). It is proposed that these imaging agents will be used for off-label indications that may provide options for disease monitoring where there are no approved tracers available, for instance Crohn’s disease or osteoporosis. Fundamental science investigations have made progress in elucidating the involvement of integrin in various diseases not pertaining to oncology. Furthermore, RGD-based radiopharmaceuticals have been evaluated extensively for safety during clinical evaluations of various natures.
Conclusion
Clinical translation of non-oncological applications for RGD-based radiopharmaceuticals and other imaging tracers without going through time-consuming extensive development is therefore highly plausible.

Graphical abstract



Similar content being viewed by others
Change history
04 March 2021
A Correction to this paper has been published: https://doi.org/10.1007/s00259-021-05264-9
References
Schwartz MA. Integrins and extracellular matrix in mechanotransduction. Cold Spring Harb Perspect Biol. 2010;2(12):a005066.
Stupack DG, Cheresh DA. Integrins and Angiogenesis. Curr Top Dev Biol. 2004;64:207–38.
Zanotelli MR, Reinhart-King CA. Mechanical forces in tumor angiogenesis. Adv Exp Med Biol. 2018;1092:91–112.
Harris AL. Hypoxia – a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2:38–47.
Cameliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307.
Chen H, Niu G, Wu H, Chen X. Clinical application of radiolabeled RGD-peptides for PET imaging of integrin αVβ3. Theranostics. 2016;6:78–92.
Debordeaux F, Chansel-Debordeaux L, Pinaquy J-B, Fernandez P, Shultz J. What about αVβ3 integrins in molecular imaging in oncology? Nucl Med Biol. 2018;62–63:31–46.
Hu G, Liu C, Liao Y, Yang L, Huang R, et al. Ultrasound molecular imaging of arterial thrombi with novel microbubbles modified by cyclic RGD in vitro and in vivo. Thromb Haemost. 2012;107:172–83.
Rojas JD, Dayton PA. In vivo molecular imaging using low-boiling-point phase-change contrast agents: a proof of concept study. Ultrasound Med Biol. 2018;45(1):177–91.
Kiessling F, Huppert J, Zhang C, Jayapaul J, Zwick S, et al. RGD-labeled USPIO inhibits adhesion and endocytotic activity of αVβ3-integrin expressing glioma cells and only accumulates in the vascular tumor compartment. Radiology. 2009;253:462–9.
Xin X, Sha H, Shen J, Zhang B, Zhu B, et al. Coupling Gd-DTPA with a bispesific recombinant protein anti-EGFR-iRGD complex improves tumor targeting in MRI. Oncol Rep. 2016;35:3227–35.
Li F, Yan H, Wang J, Li C, Wu J, et al. Non-invasively differentiating extent of liver fibrosis by visualizing hepatic integrin αVβ3 expression with an MRI modality in mice. Biomaterials. 2016;102:162–74.
Haung R, Vider J, Kovar JL, Olive DM, Mellinghoff IK, et al. Integrin αVβ3-targeted IRDye 800 CW near-infrared imaging of glioblastoma. Clin Cancer Res. 2012;8:5731–40.
Chen X, Conti PS, Moats RA. In vivo near-infrared fluorescence imaging of integrin αVβ3 in brain tumour xenografts. Cancer Res. 2004;64:8009–14.
Kwon S, Ke S, Houston JP, Wang W, Wu Q, et al. Imaging dose-dependent pharmacokinetics of an RGD-flourescent dye conjugate targeted to αVβ3 receptor expressed in Kaposi’s sarcoma. Mol Imaging. 2005;4:75–87.
Xiao Y, Zhang Q, Wang Y, Wang B, Sun F, et al. Dual-functional protein for one-step production of a soluble and targeted fluorescent dye. Theranostics. 2018;8(11):3111–25.
Choi HS, Gibbs SL, Lee JH, Kim SH, Ashitate Y, et al. Targeted zwitterionic near-infrared fluorophores for improved optical imaging. Nat Biotechnol. 2013;31:148–53.
Verbeek FPR, Van der Vorst JR, Tummer QRJG, Boonstra MC, De Rooij KE, et al. Near-infrared fluorescent imaging of both colorectal cancer and ureters using a low-dose integrin targeting probe. Ann Surg Oncol. 2014;21:528–37.
Song YS, Kim JH, Lee BC, Jung JK, Park HS, et al. Biodistribution and internal radiation dosimetry of 99mTc-IDA-D[c(RGDfK)]2(BIK-505), a novel SPECT radiotracer for imaging of integrin αVβ3 expression. Cancer Biother Radiopharm. 2018;33:396–402.
Lee BC, Moon BS, Kim JS, Jung JH, Park HS, et al. Synthesis and biological evaluation of RGD peptides with 99mTc/188Re chelated iminodiacetate core: highly enhance uptake and excretion kinetics of theranostics against tumour angiogenesis. RSC Adv. 2013;3:782–92.
Dearling JL, Barnes JW, Panigarphy D, Zimmerman RE, Fahey F, et al. Specific uptake of 99mTc-NC100692, an a αVβ3-targeted imaging probe, in subcutaneous and orthotopic tumours. Nucl Med Biol. 2013;40:788–94.
Edwards D, Jones P, Haramis H, Battle M, Lear R, et al. 99mTc-NC100692 – a tracer for imaging vitronectin receptors associated with angiogenesis: a preclinical investigation. Nucl Med Biol. 2008;35:365–75.
Jia B, Liu Z, Zhu Z, Shi J, Jin X, et al. Blood clearance kinetics, biodistribution and radiation dosimetry of a kit-formulated integrin αVβ3 selective radiotracer 99mTc-3PRGD2 in non-human primates. Mol Imaging Biol. 2011;13:730–6.
Jin X, Liang N, Wang M, Meng Y, Jia B, et al. Integrin imaging with 99mTc-3PRGD2 SPECT/CT shows high specificity in the diagnosis of lymph node metastasis from non-small cell lung can-cer. Radiology. 2016;281:958–66.
Sivolapenko GB, Skarlos D, Pectasides D, Stathopoulou E, Milonakis A, et al. Imaging of metastatic melanoma utilizing a technetium-99m labeled RGD-containing synthetic peptide. Eur J Nucl Med. 1998;25:1383–9.
Costopoulos B, Varvarigou AD, Sivolapenko G. Radiochemical and radiobiological evaluation of a synthethic peptide labeled with 99mTc [abstr]. In: Proceedings of the 8th ISORBE congress, May 1997. Castel Gandolfo, Rome Italy.
Terry SY, Abiraj K, Frielink C, Van Dijk LK, Bussink J, et al. Imaging integrin αVβ3 on blood vessels with 111In-RGD2 in head and neck tumor xenografts. J Nucl Med. 2014;55:281–6.
Terry SY, Abiraj K, Lok J, Gerrits D, Franssen GM, et al. Can 111In-RGD2 monitor respons to therapy in head and neck tumour xenografts? J Nucl Med. 2014;55:1849–55.
Haubner R, Wester HJ, Burkhart F, Senekowitch-Schmidtke R, Weber W, et al. Glycocylated RGD-containing peptides: tracer for tumor targeting and angiogenesis imaging with improved biokinetics. J Nucl Med. 2001;42:326–66.
Chen X, Park R, Shahinian AH, Bading JR, Conti PS. Pharmacokinetics and tumor retention of 125I-labeled RGD peptide are improved by PEGylation. Nucl Med Biol. 2004;31:11–9.
Hauber R, Kuhnast B, Mang C, Weber WA, Kessler H, et al. [18F]Galacto-RGD synthesis, radiolabeling, metabolic stability and radiation dose estimates. Bioconjug Chem. 2004;15:61–9.
Beer AJ, Haubner R, Wolf I, Goebel M, Luderschmidt S, et al. PET-based human dosimetry of 18F-Galacto-RGD a new radiotracer for imaging αVβ3 expression. J Nucl Med. 2006;47:763–9.
Wan W, Guo N, Pan D, Yu C, Weng Y, et al. First experience of 18F-alfatide in lung cancer patients using a new lyophilized kit for rapid radiofluorination. J Nucl Med. 2013;54:691–8.
Morrison MS, Ricketts SA, Barnett J, Cuthbertson A, Tessier J, et al. Use of a novel arg-gly-asp radioligand. 18F-AH111585 to determine changes in tumor vascularity after antitumor therapy. J Nucl Med. 2009;50:116–22.
McParland BJ, Miller MP, Spinks TJ, Kenny LM, Osmon S, et al. The biodistribution and radiation dosimetry of the Arg-Gly-asp peptide 18F-AH111585 in healthy volunteers. J Nucl Med. 2008;49:1664–7.
Chen X, Park P, Shahinian AH, Tohme M, Khankaldyyan V, et al. 18F-labeled RGD peptide: initial evaluation for imaging brain tumor angiogenesis. Nucl Med Biol. 2004;31:179–89.
Dumont RA, Hildebrandt I, Su H, Haubner R, Resichl G, et al. Noninvasive imaging of αVβ3 function as a predictor of the antimigratory and antiproliferative effects of dasatinib. Cancer Res. 2009;69:3173–9.
Lucente E, Liu H, Liu Y, Hu X, Lacivita E, et al. Novel 64Cu labeled RGD2-BBN heterotrimers for PET imaging of prostate cancer. Bioconjug Chem. 2018;29:1595–604.
Notni J, Pohle K, Wester HJ. Be spoilt for choice with radiolabeled RGD peptides: preclinical evaluation of 68Ga-TRAP(RGD)3. Nucl Med Biol. 2012;40:33–41.
Decristoforo C, Gonzalez IH, Rupprich M, Schwarz S, Virgolini I, et al. [68Ga]DOTA-RGD for the non-invasive determination of the αVβ3 integrin expression. J Nucl Med. 2006;47(S1):154P.
Kim JH, Lee JS, Kang KW, Lee HY, Han SW, et al. Whole-body distribution and radiation dosimetry of 68Ga-NOTA-RGD, a positron emission tomography agent for angiogensis imaging. Cancer Biother Radiopharm. 2012;27:65–71.
Jeong JM, Hong MK, Chang YS, Lee YS, Kim YJ, et al. Preparation of a promising angiogenesis PET imaging agent 68Ga-labeled c(RGDyK)-isothiocyanatobenzyl-1,4,7-triazacyclonanone-1,4,7-triacetic acid and feasibility studies in mice. J Nucl Med. 2008;49:830–6.
Ebenhan T, Schoeman I, Rossouw DD, Grobler A, Marjanovic-Painter B, et al. Evaluation of a flexible NOTA-RGD kit solution using gallium-68 from different 68Ge.68Ga-generators: pharmacokinetics and biodistribution in nonhuman primates and demonstration of solitary pulmonary nodule imaging in humans. Mol Imaging Biol. 2016;19:469–82.
Pohle K, Notni J, Bussemer J, Kessler H, Schwaiger M, et al. Ga-68-NODAGA-RGD is a suitable substitute for F-18-Galacto-RGD and can be produced with high specific activity in a cGMP/GRP compliant automated process. Nucl Med Biol. 2012;39:777–84.
Gnesin S, Mitsakis P, Cicone F, Deshayes E, Dunet V, et al. First in-human radiation dosimetry of 68Ga-NODAGA-RGDyK. Eur J Nucl Med Mol Imaging Res. 2017;7:43.
Satpati D, Sharma R, Kumar C, Sarma HD, Dash A. 68Ga-chelation and comparative evaluation of N,N’-bis[2-hydroxy-5-(carboxyethyl)benzyl]ethylenediamine-N,N’diacetic acid (HBED-CC) conjugated NGR and RGD peptides as tumor targeted molecular imaging probes. Med Chem Comm. 2017;8:673–9.
Knetsch PA, Zhai C, Rangger C, Blatzer M, Haas H, et al. [68Ga]FSC-(RGD)3 a trimetric RGD peptide for imaging αVβ3 integrin expression based on a novel siderophore derived chelating scaffold-synthesis and evaluation. Nucl Med Biol. 2015;42:115–22.
Knetsch PA, Petrik M, Rangger C, Seidel G, Pietzsch HJ, et al. [68Ga]NS3-RGD and [68]Oxo-DO3A-RGD for imaging α(v)β3 integrin expression: synthesis, evaluation and comparison. Nucl Med Biol. 2013;40:65–72.
Zhai C, Summer D, Rannger C, Franssen GM, Laverman P, et al. Novel biofunctional cyclic chelator of 89Zr labeling – radiolabeling and targeting properties of RGD conjugates. Mol Pharm. 2015;12:2142–50.
Hernandez R, Valdovinos HF, Yang Y, Chakravarty R, Hong H, et al. 44Sc: an attractive isotope for peptide-based PET imaging. Mol Pharm. 2014;11:2954–61.
Zang J, Mao F, Niu G, Peng L, Lang L, et al. 68Ga-BBN-RGD PET/CT for GRPR and integrin αVβ imaging in patients with breast cancer. Theranostics. 2018;8(4):1121–30.
Zang J, Li D, Lang L, Zhu Z, Wang L, et al. 68Ga-NOTA-Aca-BBN(7-14)PET/CT in healthy volunteers and glioma patients. J Nucl Med. 2016;57(1):9–14.
Lee HY, Li Z, Chen K, Hsu AR, Xu C, et al. PET/MRI dual-modality tumour imaging using arginine-glycine-aspartic (RGD)-conjugated radiolabeled iron oxide nanoparticles. J Nucl Med. 2008;49:1371–9.
Park J-A, Kim JY, Lee YJ, Lee W, Lim SM, et al. Gadolinium complex of 125I/127I-RGD-DOTA conjugate as a tumour targeting SPECT/MR bimodal imaging probe. ACS Med Chem Lett. 2013;4:216–9.
Li C, Wang W, Wu Q, Ke S, Houston J, et al. Dual optical and nuclear imaging in human melanoma xenografts using a single targeted imaging probe. Nucl Med Biol. 2006;33:349–58.
Chen Q, Wang H, Liu H, Wen S, Peng C, et al. Multifunctional dendrimer-entrapped gold nanoparticles modified with RGD peptide for targeted computed tomography/magnetic resonance dual-modal imaging of tumours. Anal Chem. 2015;87:3949–56.
Pandya DN, Hantgan R, Budzevich MM, Kock ND, Morse DL, et al. Preliminary therapy evaluation of 225Ac-DOTA-c(RGDyK) demonstrates that Cherenkov radiation derived from 225Ac daughter decay can be detected by optical imaging for in vivo tumor visualization. Theranostics. 2016;6:698–709.
Ogawa K, Takeda T, Mishiro K, Toyoshima A, Shiba K, et al. Radiotheranostics coupled between an At-211-labeled RGD peptide and the corresponding radioiodine-labeled RGD peptide. ACS Omega. 2019;4:4584–91.
Bozon-Petitprin A, Bacot S, Gauchez AS, Ahmandi M, Bourre JC, et al. Targeted radionuclide therapy with RAFT-RGD radiolabeld with 90Y or 177Lu in a mouse model of αVβ3 expressing tumours. Eur J Nucl Med Mol Imaging. 2015;42:252–63.
Yang Y, Zhang L, Cai J, Li X, Cheng D, et al. Tumour angiogenesis targeted radiosensitization therapy using gold nanoprobes guided by MRI/SPECT imaging. ACS Appl Mater Interfaces. 2016;8:1718–32.
Wang J, Zhao H, Zhou Z, Zhou P, Yan Y, et al. MR/SPECT imaging guided photothermal therapy of tumour targeting Fe@Fe3O4 nanoparticles in vivo with low mononuclear phagocyte uptake. ACS Appl Mater Interfaces. 2015;8:19872–82.
Yu G, Yang Z, Fu X, Yung BC, Yang J, et al. Polyrotaxan-based supramolecular theranostics. Nat Commun. 2018;9:766.
Huang H, Dong Y, Zhang Y, Ru D, Shihua W, et al. GSH-sensitive Pt (IV) prodrug-loaded phase-transitional nanoparticles with a hybrid lipid-polymer shell for precise theranostics against ovarian cancer. Theranostics. 2018;9(4):1047–56.
Niebler M, Reuning U, Reichart F, Notni J, Wester HJ, Schwaiger M, et al. Exploring the role of RGD-recognizing integrins in cancer. Cancers. 2017;9(9):116.
Sinanan AC, Machell JR, Wynne-Hughes GT, Hunt NP, Lewis MP. Alpha v beta 3 and alpha v beta 5 integrins and their role in muscle precursor cell adhesion. Biol Cell. 2008;100(8):465–77.
Antonov AS, Antonova GN, Munn DH, Mivechi N, Lucas R, Catravas JD, et al. αVβ3 Integrin regulates macrophage inflammatory response vis PI3 kinase/Akt-dependent NK-kB activation. J Cell Physciol. 2011;226(2):469–76.
Ward PA. Inflammation and αVβ3 integrin. Am J Respir Crit Care Med. 2012;185(1):5–6.
Hsueh WA, Law RE, Yuns S. Integrins, adhesion and cardiac remodelling. Hypertenstion. 1998;31:176–80.
Prowse ABJ, Chong F, Gray PP, Munro TP. Stem cell integrins: implications for ex-vivo culture and cellular therapies. Stem Cell Res. 2011;6(1):1–12.
Pysz MA, Gambhir SS, Willmann JK. Molecular imaging: current status and emerging strategies. Clin Radiol. 2010;65:500–16.
Wong FC, Kim EE. A review of molecular imaging studies reaching the clinical stage. Eur J Radiol. 2009;70:205–11.
Glunde K, Pathak AP, Bhujwalla ZM. Molecular-functional imaging of cancer: to image and imagine. Trends Mol Med. 2007;13(7):287–97.
Gaertner FC, Kessler H, Wester HJ, Schwaiger M, Beer AJ. Radiolabeled RGD peptides for imaging and therapy. Eur J Nucl Med Mol Imaging. 2012;39:S126–38.
Abouzied MM, Crawford ES, Nabi HA. 18F-FDG imaging: pitfalls and artifacts. J Nucl Med Technol. 2005;33:145–55.
Sun Y, Zeng Y, Zhu Y, Feng F, Xu W, et al. Application of 68Ga-PRGD2 PET/CT for αVβ3 integrin imaging of myocardial infarct and stroke. Theranostics. 2014;4:778–86.
Kim LT, Yamada KM. The regulation of expression of integrin receptors. Proc Soc Exp Biol Med. 1997;214:123–31.
Venoit JP, Srivatsa S, Carlson P. β3 integrin – a prosmicous integrin involved in vascular pathology. Can J Cardiol. 1999;15:762–70.
Ikari Y, Yee KO, Schwart SM. Role of α5β1 and αVβ3 integrins on smooth muscle cell migration in fibrin gels. Thromb Haemost. 2000;84:701–5.
Davenpeck KL, Marcinkiewiz C, Wang D, Niculescu R, Shi Y, et al. Regional differences in integrin expression: role of α5β1 in regulating smooth cell muscle functions. Circ Res. 2001;88:352–8.
Moiseeva EP. Adhesion receptors of vascular smooth muscle cells and their functions. Cardiovasc Res. 2001;52:372–86.
Al-Fakhari N, Wilhelm J, Hahn M, Heidt M, Herlein FW, et al. Increased expression of disintegrin-metalloproteinases ADAM-15 and ADAM-9 following upregulation of integrins α5β1 and αVβ3 in atherosclerosis. J Cell Biochem. 2003;89:808–23.
Lee SJ, Paeng JC. Nuclear molecular imaging for vulnerable atherosclerotic plaques. Korean J Radiol. 2015;16:955–66.
Haukkala J, Laitinen L, Luoto P, Ivenson P, Wilson I, et al. 68Ga-DOTA-RGD peptide: biodistribution and binding into atherosclerotic plaques in mice. Eur J Nucl Med Mol Imaging. 2009;36:2058–67.
Laitinen I, Saraste A, Weidl E, Poethko T, Weber AW, et al. Evaluation of αVβ3-integrin targeted positron emission tomography tracer 18F-Galacto-RGD for imaging of vascular inflammation in atherosclerotic mice. Circ Cardiovasc Imaging. 2009;2:331–8.
Razavian M, Marfatia R, Mongue-Din H, Tavakoli S, Sinusas AJ, et al. Intergin-targeted imaging of inflammation in vascular remodeling. Atheroscler Tromb Vasc Biol. 2011;31:2820–6.
Paeng JC, Lee YS, Lee JS, Jeong JM, Kim KB, et al. Feasibility and kinetic characteristics of 68Ga-NOTA-RGD PET for in vivo atherosclerosis imaging. Ann Nucl Med. 2013;27:847–54.
Beer AJ, Pelisek J, Heider P, Saraste A, Reeps C, et al. PET/CT imaging of integrin αVβ3 expression in human carotid atherosclerosis. JACC Cardiovasc Imaging. 2014;7:178–87.
Golestani R, Mirfeizi L, Zeebregts CJ, Westra J, De Haas HJ, et al. Feasibility of [18F]-RGD for ex vivo imaging of atherosclerosis in detection of αVβ3-integrin expression. J Nucl Cardiol. 2015;6:1179–86.
Reyes E. A novel PET tracer for targeted imaging of atherosclerosis. J Nucl Cardiol. 2015;22:1191–4.
Su H, Gorodny N, Gomez LF, Gangadharmath UB, Mu F, et al. Atherosclerotic plaque uptake of novel integrin tracer 18F-flotegatide in a mouse model of atherosclerosis. J Nucl Cardiol. 2014;21:531–62.
Makowski MR, Ebersberger U, Nekolla S, Schwaiger M. In vivo molecular imaging of angiogensis targeting αVβ3-integrin expression in a patient after acute myocardial infarction. Eur Heart J. 2008;29:2201.
Sherif HM, Saraste A, Nekolla SG, Weidl E, Reder S, et al. Molecular imaging of early αVβ3 integrin expression predicts long-term left-ventricle remodeling after myocardial infarction in rats. J Nucl Med. 2012;53:318–23.
Luo Y, Sun Y, Zhu ZL. Is the change of integrin αVβ3 expression in the infracted myocardium related to the clinical outcome? Clin Nucl Med. 2014;39:655–7.
Golestani R, Jung JJ, Sadeghi MM. Molecular imaging of angiogensis and vascular remodeling in cardiovascular pathology. J Clin Med. 2016;5:57.
Jenkins WS, Vesey AT, Stirrat C, Connell M, Lucatelli C, et al. Cardiac αVβ3 integrin expression following acute myocardial infarction in humans. Heart. 2017;103:607–15.
Gao H, Lang L, Guo N, Cao F, Quan Q, et al. PET imaging of angiogenesis after myocardial infarction/reperfusion using one-step labeled integrin-targeting tracer 18F-AIF-NOTA-PRGD2. Eur J Nucl Med Mol Imaging. 2012;39:683–92.
Eo JS, Paeng JC, Lee S, Lee YS, Jeong JM. Angiogenesis imaging in myocardial infarction using 68Ga-NOTA-RGD PET: characterization and application to therapeutic efficacy monitoring in rats. Coron Artery Dis. 2013;24:303–11.
Laitinen I, Dregely I, Rudelius M, Baumgartner C, Farrell E, et al. Characterization of inflammation and angiogenesis in atherosclerosis with simultaneous PET/MRI in a rabbit model. J Nucl Med. 2013;54(S2):407.
Menichetti L, Kusmic C, Panetta D, Arosio D, Petroni D, et al. Micro-PET/CT imaging of αVβ3 integrin via a novel 68Ga-NOTA-RGD peptidomimetic conjugate in rat myocardial infarction. Eur J Nucl Med Mol Imaging. 2013;40:1265–74.
Kuigel M, Dijkgraaf I, Kytö V, Helin S, Liljenbäck H, et al. Dimeric [68Ga]DOTA-RGD peptide targeting of αVβ3 integrin reveals extracellular matrix alterations after myocardial infarction. Mol Imaging Biol. 2014;16:793–801.
Paeng JC, Bregasi A, Sahul Z, Kalinowski L, Dobrucki L, et al. Serial reference tissue-based quantitative and volumetric analysis of integrin-targeted angiogenesis imaging: chronic canine model of myocardial infarction. J Nucl Med. 2014;55(S1):1710.
Kitagawa T, Kosuge H, Uchida M, Dua MM, Lida Y, et al. RGD-conjugated human ferritin nanoparticles for imaging vascular inflammation and angiogenesis in experimental carotid and aortic disease. Mol Imaging Biol. 2011;14:315–24.
Kitagawa T, Kosuge H, Uchida M, Lida Y, Dalman RL, et al. RGD targeting of human ferritin iron oxide nanoparticles enhances in vivo MRI of vascular inflammation and angiogenesis in experimental carotid disease and abdominal aortic aneurysm. J Mag Reson Imaging. 2017;45:1144–53.
Van Den Borne SW, Isobe S, Verjans JW, Petrov A, Lovaung D, et al. Molecular imaging of interstitial alterations in remodeling myocardium after myocardial infarct. J Am Col Cardiol. 2008;52:2017–28.
Wang H, Sun Y, Wu C, Zhu Z. 68Ga-BNOTA-PRGD2 PET/CT for evaluation of post-stroke angiogenesis: study protocol for a prospective open-label clinical trial. Asia Pacific J Clinc Trials: Nerv System Dis. 2016;1:43–9.
Grönman M, Tarkia M, Kiviniemi T, Halonen P, Kuivanen A, et al. Imaging of αVβ3 integrin expression in experimental myocardial ischemia with 68Ga-NODAGA-RGD positron emission tomography. J Transl Med. 2017;15:144.
Hua J, Dobrucki JW, Sadeghi MM, Zang J, Bourke BN, et al. Non-invasive imaging of angiogenesis with 99mTc-labeled peptide targeted at αVβ3 integrin after murine hindlimb ischemia. Circulation. 2005;111:3225–60.
Filardo G, Powell JT, Martinez MA, Ballard DJ. Surgery for small asymptomatic abdominal aortic aneurysms. Cochrane Database Syst Rev. 2015;3:CD001835.
Tegler G, Estrada S, Hall H, Wanhainen A, Björck M, et al. Autoradiography screening of potential positron emission tomography tracers for asymptomatic abdominal aortic aneurysms. Ups J Med Sci. 2014;119:229–35.
Stacy MR, Sinusas AJ. Novel applications of radionuclide imaging in peripheral vascular disease. Cardiol Clin. 2016;34:167–77.
Alumatari A, Rossin R, Shokeen M, Hagooly A, Anath A, et al. Biodegradable dendritic positron-emitting nanoprobes for the invasive imaging of angiogenesis. Proc Natl Acad Sci U S A. 2009;106:685–90.
Xie F, Lof J, Everbach C, He A, Bennett RM, et al. Treatment of acute intravascular thrombi with diagnostic ultrasound and intravenous microbubbles. JACC Cardiovasc Imaging. 2009;2:511–8.
Lobeek D, Bouwman FCM, Aarntzen EHJG, Molkenboer-Keunen JDM, Flucke UE, et al. A clinical feasibility study to image angiogenesis in patients with arteriovenous malformations using 68Ga-RGD PET.CT. J Nucl Med. 2019. https://doi.org/10.2967/jnumed.119.231167.
Kim YI, Phi JH, Paeng JC, Choi H, Kim SK, et al. In vivo evaluation of angiogenic activity and its correlation with efficacy of indirect revascularization surgery in paediatric moyamoya disease. J Nucl Med. 2014;55:1467–72.
Kuroda S, Houkin K. Moyamoya disease: current concepts and future perspectives. Lancet Neurol. 2008;7:1056–66.
Ocak M, Demirci E, Day K, Latoche J, Liu J, et al. Evaluation of 64Cu and 68Ga-NODAGA-c(RGDyK) in a rodent inflammatory paw model. J Nucl Med. 2014;55(S1):1222.
Cao Q, Cai W, Li ZB, Chen K, He L, et al. PET imaging of acute and chronic inflammation in living mice. Eur J Nucl Med Mol Imaging. 2007;34:1832–42.
Pichler BJ, Kneilling M, Haubner R, Bramüller H, Schwaiger M, et al. Imaging of delayed-type hypersensitivity reaction by PET and 18F-Galacto-RGD. J Nucl Med. 2005;46:184–9.
Shu S, Zhang L, Zhu YC, Li F, Cui LY, et al. Imaging angiogenesis using 68Ga-NOTA-PRGD2 positron emission tomography/computed tomography in patients with severe intracranial atherosclerotic disease. J Cereb Blood Flow Metab. 2017;37:3401–8.
Zheleznyak A, Wadas TJ, Sherman CD, Wilson JM, Kostenuik PJ, et al. Integrin αVβ3 as a PET imaging biomarker for osteoclast number in mouse models of negative and positive osteoclast regulation. Mol Imaging Biol. 2012;14:500–8.
Wilder RL. Integrin alpha V beta 3 as a target for treatment of rheumatoid arthritis and related rheumatic disease. Ann Rheum Dis. 2002;61(S2):ii96–9.
Notni J, Gassert FT, Steiger K, Sommer P, Wichert W, et al. In vivo imaging of early stages of rheumatoid arthritis by α5β1-integrin-targeted positron emission tomography. EJNNMI Res. 2019;9:87.
Bhatnagar S, Khera E, Liao J, Eniola V, Hu Y, et al. Oral and subcutaneous administration of a near-infrared fluorescent molecular imaging agent detects inflammation in a mouse model of rheumatoid arthritis. Sci Rep. 2019;9:4661.
Chen DL, Schieldbler ML, Goo JM, Van Beek EJR. PET imaging approaches for inflammatory lung diseases: current concepts and future directions. Eur J Radiol. 2017;86:371–6.
Mirsadree S, Marin A, Jenkins W, Connell T, Tavares A, et al. The identification of systemic integrin activation in idiopathic and systemic sclerosis pulmonary fibrosis using 18F-fluciclatide positron emission tomography. Insights Imag. 2016;7:S424.
Schniering J, Benesova M, Brunner M, Haller S, Chors S, et al. Visualisation of interstitial lung disease by molecular imaging of integrin αvβ3 and somatostatin receptor 2. Ann Rheum Dis. 2019;78(2):218–27.
Shao T, Josephson L, Liang SH. PET/SPECT molecular probes for the diagnosis and staging of non-alcoholic fatty liver disease. Mol Imaging. 2019;18:1–11.
Pozzi A, Zent R. Integrins in kidney disease. J Am Soc Neph. 2013;24:1034–9.
Du Y, An S, Liu L, Li L, Zhou XJ, et al. Serial non-invasive monitoring of renal disease following immune-mediated injury using near-infrared optical imaging. PLoS One. 2012;7:e43931.
Ferrara JLM, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;73:1550–61.
Deininger F, Leonhard F, Fani M, Dumont R, Toennesmann R, et al. PET imaging of inflammatory processes during GvHD. J Nucl Med. 2011;52(S1):518.
Mozid AM, Holstensson M, Choudhury T, Ben-Haim S, Allie R, et al. Clinical feasibility study to detect angiogenesis following bone marrow stem cell transplantation in chronic ischaemic heart failure. Nucl Med Commun. 2014;35:839–48.
Nguyen PK, Lan F, Wang Y, Wu JC. Imaging guiding the clinical translation of cardiac stem cell therapy. Circ Res. 2011;109:962–79.
Evens P, McParland B, Zubeldia J. Application of 99mTc peptide-based compound as bone marrow imaging agent. https://patents.google.com/patent/US20110256055A1/en. US20110256055A1.
Nam YS, Ricles LM, Suggs LJ, Emalianov SY. Imaging strategies for tissue engineering applications. Tissue Eng B. 2015;21:88–102.
Shachar M, Tsur-gang O, Dvir T, Leor J, Cohen S. The effect of immobilized RGD peptide in alginate scaffolds on cardiac tissue engineering. Acta Biomater. 2011;7:152–62.
Zheng W, Wang Z, Song L, Zhao Q, Zhang J, et al. Endothelialization and patency of RGD-functionalized vascular grafts in a rabbit carotid artery model. Biomaterials. 2012;33:2280–91.
Hennessy KM, Clem WC, Phillips MC, Sawyer AA, Shaikh FM, et al. The effect of RGD peptides on osseointegration of hydroxyapatite biomaterials. Biomaterials. 2008;29:3075–83.
Wholrab S, Müller S, Schmidt A, Neubauer S, Kessler H, et al. Cell adhesion and proliferation on RGD-modified recombinant spider silk proteins. Biomaterials. 2012;33:6650–9.
Patra C, Ricciardia F, Engel FB. The functional properties of nephronectin: an adhesion molecule for cardiac tissue engineering. Biomaterials. 2012;33:4327–35.
Chin CW, Vassiliou V, Jenkins WS, Prasad SK, Newby DE, et al. Markers of left ventricular decompensation in aortic stenosis. Expert Rev Cardiovasc Ther. 2014;12:901–12.
Serini G, Valdembri D, Bussolino F. Integrins and angiogenesis: a sticky business. Exp Cell Res. 2006;312:651–8.
Lygoe KA, Norman JT, Marshall JF, Lewis MP. Alpha V integrins play an important role in myofibroblast differentiation. Wound Repair Regen. 2004;12:461–70.
Su G, Atakilit A, Li JT, Wu N, Bhattacharya M, et al. Absence of ingetrin αVβ3 enhances vascular leak in mice by inhibiting endothelial cortical actin formation. Am J Respir Crit Care Med. 2012;185:58–66.
Danese S, Sans M, De la Motte C, Graziani C, West G, et al. Angiogenesis as a novel component of inflammatory bowel disease pathogenesis. Gastroenterol. 2006;130:2060–73.
Aziz MM, Ishihara S, Mishima Y, Oshima N, Moriyama I, et al. MFG-E8 attenuates intestinal inflammation in murine experimental colitis by modulating osteopontin-dependents alpha V beta 3 integrin signaling. J Immunol. 2009;182:7222–32.
Roivainen A, Jalkanen S, Nanni C. Gallium-labelled peptides for imaging of inflammation. Eur J Nucl Med Mol Imaging. 2012;39:S68–77.
Zhao H, Kitaura H, Sands MS, Ross FP, Teitelbaum SL, et al. Critical role of β3 integrin in experimental postmenopausal osteoporosis. J Bone Miner Res. 2005;20:2116–23.
Murphy MG, Cerchio K, Stoch SA, Gottesdiener K, Wu M, et al. Effect of L-000845704, an alphaVbeta3 integrin antagonist, on markers of bone turnover and bone mineral density in postmenopausal osteoporotic women. J Clin Endocrinol Metab. 2005;90:2022–8.
Lin TH, Yang RS, Tu HJ, Liou HC, et al. Inhibition of osteoporosis by the αVβ3 integrin antagonist of rhodostomin variants. Eur J Pharmacol. 2017;804:94–101.
Oei L, Koromani F, Rivadeneira F, Zillikens MC, Oei EHG. Quantitative imaging methods in osteoporosis. Quant Imaging Med Surg. 2016;6:680–98.
Roth T, Podestá F, Stepp MA, Boeri D, Lorenzi M. Integrin overexpression induced by high glucose and by human diabetes: potential pathway of cell dysfunction in diabetic microangiopathy. Proc Natl Acad Sci U S A. 1993;90:9640–4.
Regoli M, Bendayan M. Alterations in the expression of the α3β1 integrin in certain membrane domains of the glomerular epithelial cells (podocytes) in diabetes mellitus. Diabetologia. 1997;40:15–22.
Metha NN, Yu Y, Saboury B, Foroughi N, Krishnamoorthy P, et al. Systemic and vascular inflammation in patients with moderate to severe psoriasis as measured by [18F]-fluorodeoxyglucose postiron emission tomography-computed tomography (FDG PET/CT): a pilot study. Arch Dermatol. 2011;147:1031–9.
Takata T, Taniguchi Y, Ohnishi T, Kohsaki S, Nogami M, et al. 18FDG PET/CT is a powerful tool for detecting subclinical arthritis in patients with psoriatic arthritis and/or psoriasis vulgaris. J Dermatol Sci. 2011;64:144–7.
Bains S, Reimert M, Win AZ, Khan S, Aparici CM. A patient with psoriatic arthritis imaged with FDG-PET/CT demonstrated an unusual imaging pattern with muscle and facia involvement: a case report. Nucl Med Mol Imaging. 2012;46:138–43.
Jókai H, Szakonyi J, Kontár O, Marschalkó M, Szalai K, et al. Impact of the effective tumor necrosis factor alpha inhibitor treatment on the arterial intimia media thickness in psoriasis: results of a pilot study. J Am Acad Dermatol. 2013;69:523–39.
Naik HB, Natarajan B, Stansky E, Ahlman MA, Teague H, et al. Severity of psoriasis associates with aortic vascular inflammation detected by FDG PET/CT and neutrophil activation in a prospective observational study. Atheroscler Thromb Vasc Biol. 2015;35:2667–76.
Creamer D, Allen M, Sousa A, Poston R, Barker J. Altered vascular endothelium integrin expression in psoriasis. Am J Pathol. 1995;147:1661–7.
Garrigues HJ, Rubinchikova YE, DiPersio CM, Rose TM. Integrin αVβ3 binds to the RGD motif of glycoprotein B of Kaposi’s sarcoma-associated herpes virus and functions as a RGD-dependent entry receptor. J Virol. 2008;82:1570–80.
O’Donell V, Pacheco JM, Gregg D, Baxt B. Analysis of foot-and-mouth disease virus integrin receptor expression in tissues from naïve and infected cattle. J Comp Pathol. 2009;141:98–112.
Antonova LV, Silnikov VN, Sevostyanova VV, Yuzhalin AE, Koroleva LS, Velikanova EA, et al. Biocompatibility of small-diameter vascular grafts in different modes of RGD modification. Polymers. 2020;11(1):174. https://doi.org/10.3390/polym11010174.
Alipour M, Baneshi M, Hosseinkhani S, Mahmoudi R, Arabzadeh AJ, Akrami M, et al. Recent progress in biomedical applications of RGD-based ligand: from precise cancer theranostics to biomaterial engineering: a systematic review. J Biomed Mater Res A. 2020:108(4). https://doi.org/10.1002/jbm.a36862.
Tahlawi A, Klontzas ME, Allenby MC, Morais JCF, Panoskaltisis N, Mantalaris A. RGD-functionalised polyurethane scaffolds promote umbilical cord blood mesenchymal stem cell expansion and osteogenic differentiation. J Tissue Eng Regen Med. 2018:13(2). https://doi.org/10.1002/term.2784.
Liu Y, Ma LT, Zhou H, Qianqian Y, Chen X, et al. Polypeptide nano-se targeting inflammation and theranostic rheumatoid arthritis by anti-angiogenic and NO activating AMPKα signaling pathway. J Mater Chem B. 2018;6:3497–514.
Tang M, Ji X, Xu H, Zhang L, Jiang A, et al. Photostable and biocompatible fluorescent silicon nanoparticles-based theranostic probes for simultaneous imaging and treatment of ocular neovascularization. Anal Chem. 2018;90:8188–95.
Horton MA. The αVβ3 integrin “vironectin receptor”. Int J Biochem Cell Biol. 1997;29:721–5.
Wang Y, Liu Z, Li T, Chen L, Lyu J, et al. Enhanced therapeutic effect of RGD-modified polymeric micelles loaded with low-dose methrotrexate and nimesulide on rheumatoid arthritis. Theranostics. 2018;9(3):708–20.
Knapp FF, Dash A. Translation of radiopharmaceuticals from bench to bedside: regulatory and manufacturing issues. Radiopharmaceuticals for therapy – Chapter 17: 323–343. Springer. https://doi.org/10.1007/978-81-322-2607-9_17.
Peitl PK, Rangger C, Garnuszek P, Mikolajczak R, Hubalewska-Dydejcsyk A, Maina T, et al. Clinical translation of theranostic radiopharmaceuticals: current regulatory status and recent examples. J Labelled Comp Radiopharm. 2019;62(10):673–83. https://doi.org/10.1002/jlcr.3712.
Resichl G. Special issue “targets, tracers and translation novel radiopharmaceuticals boost nuclear medicine”. Pharmaceuticals. 2019;12:111. https://doi.org/10.3390/ph12030111.
Allot L, Oboagye EO. Chemistry considerations for the clinical translation of oncology PET radiopharmaceuticals. Molecular Pharmaceuticals. 2020. (In press): https://doi.org/10.1021/acs.molpharmaceut.0c00328.
Acknowledgments
The graphic abstract for this article was provided by the scientific illustrator Cristina Sala Ripoll (www.cristinasalaripoll.com). Language editing was kindly provided by Hester Oosthuizen (hester.oos@mweb.co.za), and advanced language editing and scientific editing was provided by Bioeditman (http://bioeditman.org/contact-5/).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no conflict of interest.
Related manuscripts
The authors declare that no related manuscripts are under consideration for publication elsewhere.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: There was a mistake in the original article. The references cited in Table 1 to Table 4 are incorrect. The correct set of references per table appears below. Table 1 must rather refer to references 17 (first entry) to 27 (last entry) NOT 8 (first) to 18 (last) Table 2 must rather refer to references 28 (first entry) to 61 (last entry) NOT 19 (first) to 52 (last) Table 3 must rather refer to references 62 (first entry) to 65 (last entry) NOT 53 (first) to 56 (last) Table 4 must rather refer to references 66 (first entry) to 72 (last entry) NOT 57 (first) to 63 (last) The original article has been corrected.
This article is part of the Topical Collection on Cardiology
Rights and permissions
About this article
Cite this article
Ebenhan, T., Kleynhans, J., Zeevaart, J.R. et al. Non-oncological applications of RGD-based single-photon emission tomography and positron emission tomography agents. Eur J Nucl Med Mol Imaging 48, 1414–1433 (2021). https://doi.org/10.1007/s00259-020-04975-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-020-04975-9