Abstract
Purpose
To study the feasibility of the in vivo [18F]-DPA-714 TSPO positron emission tomography (PET) to detect glial activation in a rat model of progressive parkinsonism induced by viral-mediated overexpression of A53T mutated human α-synuclein (hα-syn) in the substantia nigra pars compacta (SNpc).
Methods
We conducted a cross-sectional study in a model of progressive parkinsonism. Bilateral intranigral injections with 2/9 adeno-associated viral vectors encoding either hα-syn (AAV-hα-syn) or green fluorescent protein (AAV-GFP) were performed in rats (n = 60). In vivo [18F]-DPA-714 PET imaging was performed at different time points after inoculation (p.i.) of the viral vector (24 and 72 h and 1, 2, 3, and 16 weeks). Images were analyzed to compute values of binding potential (BP) in the SNpc and striatum using a volume of interest (VOI) analysis. Immunohistochemistry of markers of dopaminergic degeneration (tyrosine hydroxylase (TH)), microglia (Iba-1), and astrocytes (GFAP) was carried out. Binding potential (BP) of [18F]-DPA-714 PET in the in vivo PET study was correlated with post-mortem histological markers.
Results
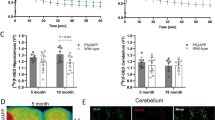
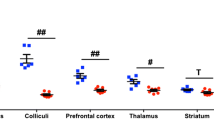
In the SNpc of AAV-hα-syn rats, there was higher in vivo [18F]-DPA-714 BP (p < 0.05) and increased number of post-mortem Iba-1+ cells (p < 0.05) from second week p.i. onwards, which were highly correlated (p < 0.05) between each other. These findings antedated the nigral reduction of TH+ cells that occurs since third week p.i. (p < 0.01). In addition, the [18F]-DPA-714 BP was inversely correlated (p < 0.05) with the TH+ cells. In contrast, GFAP+ cells only increased at 16 weeks p.i. and did not correlate with the in vivo results. In the striatum, no changes in the number of Iba-1+ and GFAP+ cells were observed, but an increment in the [18F]-DPA-714 BP was found at 16 weeks p.i.
Conclusions
Our study showed that in vivo PET study with [18F]-DPA-714 is a selective and reliable biomarker of microglial activation and could be used to study preclinical stages of Parkinson’s disease (PD) and to monitor the progression of the disease.





Similar content being viewed by others
References
Czeh M, Gressens P, Kaindl AM. The yin and yang of microglia. Dev Neurosci. 2011;33:199–209.
Chen M-K, Guilarte TR. Translocator protein 18 kDa (TSPO): molecular sensor of brain injury and repair. Pharmacol Ther. 2008;118:1–17.
Venneti S, Lopresti BJ, Wiley CA. The peripheral benzodiazepine receptor (translocator protein 18 kDa) in microglia: from pathology to imaging. Prog Neurobiol. 2006;80:308–22.
Chauveau F, Boutin H, Van Camp N, Dollé F, Tavitian B. Nuclear imaging of neuroinflammation: a comprehensive review of [11C]PK11195 challengers. Eur J Nucl Med Mol Imaging. 2008;35:2304–19.
Venneti S, Lopresti BJ, Wiley CA. Molecular imaging of microglia/macrophages in the brain. Glia. 2013;61:10–23.
Bartels AL, Willemsen ATM, Doorduin J, de Vries EFJ, Dierckx RA, Leenders KL. [11C]-PK11195 PET: quantification of neuroinflammation and a monitor of anti-inflammatory treatment in Parkinson’s disease? Parkinsonism Relat Disord. 2010;16:57–9.
Ouchi Y, Yoshikawa E, Sekine Y, Futatsubashi M, Kanno T, Ogusu T, et al. Microglial activation and dopamine terminal loss in early Parkinson’s disease. Ann Neurol. 2005;57:168–75.
Gerhard A, Pavese N, Hotton G, Turkheimer F, Es M, Hammers A, et al. In vivo imaging of microglial activation with [11C](R)-PK11195 PET in idiopathic Parkinson’s disease. Neurobiol Dis. 2006;21:404–12.
Boutin H, Chauveau F, Thominiaux C, Grégoire MC, James ML, Trebossen R, et al. 11C-DPA-713: a novel peripheral benzodiazepine receptor PET ligand for in vivo imaging of neuroinflammation. J Nucl Med. 2007;48:573–81.
Venneti S, Wang G, Wiley CA. The high affinity peripheral benzodiazepine receptor ligand DAA1106 binds to activated and infected brain macrophages in areas of synaptic degeneration: implications for PET imaging of neuroinflammation in lentiviral encephalitis. Neurobiol Dis. 2008;29:232–41.
Chauveau F, Van Camp N, Dollé F, Kuhnast B, Hinnen F, Damont A, et al. Comparative evaluation of the translocator protein radioligands 11C-DPA-713, 18F-DPA-714, and 11C-PK11195 in a rat model of acute neuroinflammation. J Nucl Med. 2009;50:468–76.
Terada T, Yokokura M, Yoshikawa E, Futatsubashi M, Kono S, Konishi T, et al. Extrastriatal spreading of microglial activation in Parkinson’s disease: a positron emission tomography study. Ann Nucl Med Springer Japan. 2016;30:579–87.
Varnäs K, Cselényi Z, Jucaite A, Halldin C, Svenningsson P, Farde L, et al. PET imaging of [11 C]PBR28 in Parkinson’s disease patients does not indicate increased binding to TSPO despite reduced dopamine transporter binding. Eur J Nucl Med Mol Imaging. 2019;46:367–75.
Ghadery C, Koshimori Y, Coakeley S, Harris M, Rusjan P, Kim J, et al. Microglial activation in Parkinson’s disease using [18F]-FEPPA. J Neuroinflammation [Internet]. J Neuroinflammation; 2017;14:1–9. Available from: https://doi.org/10.1186/s12974-016-0778-1
Mabrouk R, Strafella AP, Knezevic D, Ghadery C, Mizrahi R, Gharehgazlou A, et al. Feasibility study of TSPO quantification with [18F]FEPPA using population-based input function. PLoS One. 2017;12(5):e0177785. https://doi.org/10.1371/journal.pone.0177785. eCollection 2017.
Golla SSV, Boellaard R, Oikonen V, Hoffmann A, van Berckel BNM, Windhorst AD, et al. Quantification of [18F]DPA-714 binding in the human brain: initial studies in healthy controls and Alzheimer’s disease patients. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 2015;35:766–72.
Hamelin L, Lagarde J, Dorothée G, Leroy C, Labit M, Comley RA, et al. Early and protective microglial activation in Alzheimer’s disease: a prospective study using 18F-DPA-714 PET imaging. Brain. 2016;139:1252–64.
Corcia P, Tauber C, Vercoullie J, Arlicot N, Prunier C, Praline J, et al. Molecular imaging of microglial activation in amyotrophic lateral sclerosis. PLoS One. 2012;7:e52941.
Fricke IB, Viel T, Worlitzer MM, Collmann FM, Vrachimis A, Faust A, et al. 6-hydroxydopamine-induced Parkinson’s disease-like degeneration generates acute microgliosis and astrogliosis in the nigrostriatal system but no bioluminescence imaging-detectable alteration in adult neurogenesis. Eur J Neurosci. 2016;43:1352–65.
Joers V, Tansey MG, Mulas G, Carta AR. Microglial phenotypes in Parkinson’s disease and animal models of the disease. Prog Neurobiol. 2017;155:57–75.
Crabbé M, Van der Perren A, Kounelis S, Lavreys T, Bormans G, Baekelandt V, et al. Temporal changes in neuroinflammation and brain glucose metabolism in a rat model of viral vector-induced α-synucleinopathy. Exp Neurol [Internet] Elsevier. 2019;320:112964. Available from:. https://doi.org/10.1016/j.expneurol.2019.112964.
Rojas S, Martín A, Arranz MJ, Pareto D, Purroy J, Verdaguer E, et al. Imaging brain inflammation with [11C]PK11195 by PET and induction of the peripheral-type benzodiazepine receptor after transient focal ischemia in rats. J Cereb Blood Flow Metab. 2007;27:1975–86.
Ji B, Maeda J, Sawada M, Ono M, Okauchi T, Inaji M, et al. Imaging of peripheral benzodiazepine receptor expression as biomarkers of detrimental versus beneficial glial responses in mouse models of Alzheimer’s and other CNS pathologies. J Neurosci. 2008;28:12255–67.
Lavisse S, Guillermier M, Hérard AS, Petit F, Delahaye M, Van Camp NV, et al. Reactive astrocytes overexpress TSPO and are detected by TSPO positron emission tomography imaging. J Neurosci. 2012;32:10809–18.
Jiménez-Urbieta H, Gago B, Quiroga-Varela A, Rodríguez-Chinchilla T, Merino-Galán L, Oregi A, et al. Pramipexole-induced impulsivity in mildparkinsonian rats: a model of impulse control disorders in Parkinson’s disease. Neurobiol Aging. 2019;75:126–135. https://doi.org/10.1016/j.neurobiolaging.2018.11.021.
Martín A, Boisgard R, Thézé B, Van Camp N, Kuhnast B, Damont A, et al. Evaluation of the PBR/TSPO radioligand [(18)F]DPA-714 in a rat model of focal cerebral ischemia. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 2010;30:230–41.
Colás L, Domercq M, Ramos-Cabrer P, Palma A, Gómez-Vallejo V, Padro D, et al. In vivo imaging of Α7 nicotinic receptors as a novel method to monitor neuroinflammation after cerebral ischemia. Glia. 2018;66:1611–24.
Paxinos G, Watson C. The rat brain in stereotaxic coordinates. London: Acad Press; 2009. p. 456.
Schweinhardt P, Fransson P, Olson L, Spenger C, Andersson JLR. A template for spatial normalisation of MR images of the rat brain. J Neurosci Methods. 2003;129:105–13.
Frumberg DB, Fernando MS, Lee DE, Biegon A, Schiffer WK. Metabolic and behavioral deficits following a routine surgical procedure in rats. Brain Res. 2007;1144:209–18.
Ichise M, Liow JS, Lu JQ, Takano A, Model K, Toyama H, et al. Linearized reference tissue parametric imaging methods: application to [11C]DASB positron emission tomography studies of the serotonin transporter in human brain. J Cereb Blood Flow Metab. 2003;23:1096–112.
Olsson M, Nikkhah G, Bentlage C, Björklund A. Forelimb akinesia in the rat Parkinson model: differential effects of dopamine agonists and nigral transplants as assessed by a new stepping test. J Neurosci. 1995;15:3863–75.
Gundersen HJG, Jensen EBV, Kiêu K, Nielsen J. The efficiency of systematic sampling in stereology - reconsidered. J Microsc. 1999;193:199–211.
West MJ. Stereological methods for estimating the total number of neurons and synapses: issues of precision and bias. Trends Neurosci. 1999;22:51–61.
Sanchez-Guajardo V, Febbraro F, Kirik D, Romero-Ramos M. Microglia acquire distinct activation profiles depending on the degree of α-synuclein neuropathology in a rAAV based model of Parkinson’s disease. PLoS One. 2010;5:e8784.
Iannaccone S, Cerami C, Alessio M, Garibotto V, Panzacchi A, Olivieri S, et al. In vivo microglia activation in very early dementia with Lewy bodies, comparison with Parkinson’s disease. Parkinsonism Relat Disord. 2013;19:47–52.
Stokholm MG, Iranzo A, Østergaard K, Serradell M, Otto M, Svendsen KB, et al. Assessment of neuroinflammation in patients with idiopathic rapid-eye-movement sleep behaviour disorder: a case-control study. Lancet Neurol. 2017;16:789–96.
Kitamura Y, Inden M, Minamino H, Abe M, Takata K, Taniguchi T. The 6-hydroxydopamine-induced nigrostriatal neurodegeneration produces microglia-like NG2 glial cells in the rat substantia nigra. Glia. 2010;58:1686–700.
Walsh S, Finn DP, Dowd E. Time-course of nigrostriatal neurodegeneration and neuroinflammation in the 6-hydroxydopamine-induced axonal and terminal lesion models of Parkinson’s disease in the rat. Neuroscience. 2011;175:251–61.
Saxena S, Caroni P. Selective neuronal vulnerability in neurodegenerative diseases: from stressor thresholds to degeneration. Neuron [Internet] Elsevier Inc. 2011;71:35–48. Available from:. https://doi.org/10.1016/j.neuron.2011.06.031.
Galvin JE. Interaction of alpha-synuclein and dopamine metabolites in the pathogenesis of Parkinson’s disease: a case for the selective vulnerability of the substantia nigra. Acta Neuropathol. 2006;112:115–26.
Chan CS, Gertler TS, Surmeier DJ. A molecular basis for the increased vulnerability of substantia nigra dopamine neurons in aging and Parkinson’s disease. Mov Disord. 2010;25.
Bachiller S, Jiménez-Ferrer I, Paulus A, Yang Y, Swanberg M, Deierborg T, et al. Microglia in neurological diseases: a road map to brain-disease dependent-inflammatory response. Front Cell Neurosci. 2018;12:488. https://doi.org/10.3389/fncel.2018.00488. eCollection 2018.
Lawson LJ, Perry VH, Dri P, Gordon S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience. 1990;39:151–70.
Savchenko VL, Nikonenko IR, Skibo GG, McKanna JA. Distribution of microglia and astrocytes in different regions of the normal adult rat brain. Neurophysiology. 1997;29:343–51.
Vivash L, O’Brien TJ. Imaging microglial activation with TSPO PET: lighting up neurologic diseases? J Nucl Med. 2016;57:165–8.
Acknowledgments
The authors wish to thank the Molecular Imaging Unit and Animal Facility staff of CIC biomaGUNE for their excellent work in the radiotracer production and image acquisition. The imaging work was carried out at the Unique Scientific and Technical Infrastructure (ICTS) ReDIB.
Funding
This study was funded by the CIBERNED (INTRACIBER 2014/06). In addition, TR-C and AQ-V were funded by CIBERNED funds. TR-C held a Fundación Jesús de Gangoiti Barrera Foundation grant (Bilbao, Spain). HJ-U and AB-I holds a Predoctoral Research Fellowship from the Government of the Basque Country. LM-G holds a Predoctoral Research Fellowship from the Basque Country University (UPV/EHU).
Author information
Authors and Affiliations
Contributions
MCR-O, AQ-V, and BG contributed to the study conception and design. Animal model and data collection were performed by TR-C and AQ-V. PET image analyses were performed by FM-D. Histological study was performed by TR-C, and AB-I and MCR-O. supervised all the study. TR-C, AQ-V and FM-D performed the statistical analysis. AQ-V and MCR-O interpreted the results of the analysis with substantial contribution from all the authors; TR-C, AQ-V, and MCR-O drafted the manuscript, to which all the authors contributed with revisions in the previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
TR-C, AQ-V, FM-D, AB-I, LM-G, HJ-U, and BG have no disclosures to declare. MCR-O received honoraria for lectures, travel, and accommodation to attend scientific meetings from Abbvie, Bial, and Boston Scientific, and she received financial support for her research from national and local government funding agencies in Spain (Institute of Health Carlos III, Basque Country Local Government, and CIBERNED). None of these bodies influenced the content of the manuscript or the decision to publish in any way.
Ethical approval
All the procedures for the care and use of animals were approved by the ethics committees for animal research at Biodonostia Health Research Institute, CIC biomaGUNE (San Sebastián, Spain) and local authorities and were conducted in accordance with the guidelines of the Spanish Government (RD53/2013) and the European Union Council Directive (2010/63/EU) on animal ethics and welfare.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Neurology
Electronic supplementary material
ESM 1
(DOCX 169 kb)
Rights and permissions
About this article
Cite this article
Rodríguez-Chinchilla, T., Quiroga-Varela, A., Molinet-Dronda, F. et al. [18F]-DPA-714 PET as a specific in vivo marker of early microglial activation in a rat model of progressive dopaminergic degeneration. Eur J Nucl Med Mol Imaging 47, 2602–2612 (2020). https://doi.org/10.1007/s00259-020-04772-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-020-04772-4