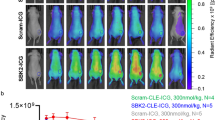
Abstract
Purpose
OTS514 is a highly specific inhibitor targeting lymphokine-activated killer T cell-originated protein kinase (TOPK). A fluorescently labeled TOPK inhibitor could be used for tumor delineation or intraoperative imaging, potentially improving patient care.
Methods
Fluorescently labeled OTS514 was obtained by conjugating the fluorescent small molecule NBD to the TOPK inhibitor. HCT116 colorectal cancer cells were used to generate tumors in NSG mice for in vivo studies. Images were generated in vitro using confocal microscopy and ex vivo using an IVIS Spectrum.
Results
OTS514 was successfully conjugated to a fluorescent sensor and validated in vitro, in vivo, and ex vivo. The labeling reaction led to TOPKi-NBD with 67% yield and 97% purity after purification. We were able to test binding properties of TOPKi-NBD to its target, TOPK, and compared them to the precursor inhibitor. EC50s showed similar target affinities for TOPKi-NBD and the unlabeled OTS514. TOPKi-NBD showed specific tumor uptake after systemic administration and was microscopically detectable inside cancer cells ex vivo. Blocking controls performed with an excess of the unlabeled OTS514 confirmed specificity of the compound. Overall, the results represent a first step toward the development of a class of TOPK-specific fluorescent inhibitors for in vivo imaging and tumor delineation.
Conclusions
TOPK has the potential to be a new molecular target for cancer-specific imaging in a large variety of tumors. This could lead to broad applications in vitro and in vivo.




Similar content being viewed by others
References
Abe Y, Matsumoto S, Kito K. Ueda N Cloning and expression of a novel MAPKK-like protein kinase, lymphokine-activated killer T-cell-originated protein kinase, specifically expressed in the testis and activated lymphoid cells. J Biol Chem. 2000;275:21525–31.
Gaudet S, Branton D. Lue RA Characterization of PDZ-binding kinase, a mitotic kinase. Proc Natl Acad Sci U S A. 2000;97:5167–72.
Shinde SR, Gangula NR, Kavela S, Pandey V. Maddika S TOPK and PTEN participate in CHFR mediated mitotic checkpoint. Cell Signal. 2013;25:2511–7.
Park JH, Lin ML, Nishidate T, Nakamura Y. Katagiri T PDZ-binding kinase/T-LAK cell-originated protein kinase, a putative cancer/testis antigen with an oncogenic activity in breast cancer. Cancer Res. 2006;66:9186–95.
Zhu F, Zykova TA, Kang BS, Wang Z, Ebeling MC, Abe Y, et al. Bidirectional signals transduced by TOPK-ERK interaction increase tumorigenesis of HCT116 colorectal cancer cells. Gastroenterology. 2007;133:219–31.
Su TC, Chen CY, Tsai WC, Hsu HT, Yen HH, Sung WW, et al. Cytoplasmic, nuclear, and total PBK/TOPK expression is associated with prognosis in colorectal cancer patients: a retrospective analysis based on immunohistochemistry stain of tissue microarrays. PLoS One. 2018;13:e0204866.
Hu F, Gartenhaus RB, Zhao XF, Fang HB, Minkove S, Poss DE, et al. c-Myc and E2F1 drive PBK/TOPK expression in high-grade malignant lymphomas. Leuk Res. 2013;37:447–54.
Simons-Evelyn M, Bailey-Dell K, Toretsky JA, Ross DD, Fenton R, Kalvakolanu D, et al. PBK/TOPK is a novel mitotic kinase which is upregulated in Burkitt’s lymphoma and other highly proliferative malignant cells. Blood Cells Mol Dis. 2001;27:825–9.
Uchida E, Suwa S, Yoshimoto R, Watanabe K, Kasama T, Miura O, et al. TOPK is regulated by PP2A and BCR/ABL in leukemia and enhances cell proliferation. Int J Oncol. 2019;54:1785–96.
Ikeda Y, Park J-H, Miyamoto T, Takamatsu N, Kato T, Iwasa A, et al. T-LAK Cell-Originated Protein Kinase (TOPK) as a Prognostic Factor and a Potential Therapeutic Target in Ovarian Cancer. Clin Cancer Res. 2016;22:6110.
Lei B, Qi W, Zhao Y, Li Y, Liu S, Xu X, et al. PBK/TOPK expression correlates with mutant p53 and affects patients’ prognosis and cell proliferation and viability in lung adenocarcinoma. Hum Pathol. 2015;46:217–24.
Shih MC, Chen JY, Wu YC, Jan YH, Yang BM, Lu PJ, et al. TOPK/PBK promotes cell migration via modulation of the PI3K/PTEN/AKT pathway and is associated with poor prognosis in lung cancer. Oncogene. 2012;31:2389–400.
Stangeland B, Mughal AA, Grieg Z, Sandberg CJ, Joel M, Nygård S, et al. Combined expressional analysis, bioinformatics and targeted proteomics identify new potential therapeutic targets in glioblastoma stem cells. Oncotarget. 2015;6:26192–215.
Quan C, Xiao J, Duan Q, Yuan P, Xue P, Lu H, et al. T-lymphokine-activated killer cell-originated protein kinase (TOPK) as a prognostic factor and a potential therapeutic target in glioma. Oncotarget. 2018;9:7782–95.
Brown-Clay JD, Shenoy DN, Timofeeva O, Kallakury BV, Nandi AK. Banerjee PP PBK/TOPK enhances aggressive phenotype in prostate cancer via β-catenin-TCF/LEF-mediated matrix metalloproteinases production and invasion. Oncotarget. 2015;6:15594–609.
Sun H, Zhang L, Shi C, Hu P, Yan W, Wang Z, et al. TOPK is highly expressed in circulating tumor cells, enabling metastasis of prostate cancer. Oncotarget. 2015;6:12392–404.
Zlobec I, Molinari F, Kovac M, Bihl MP, Altermatt HJ, Diebold J, et al. Prognostic and predictive value of TOPK stratified by KRAS and BRAF gene alterations in sporadic, hereditary and metastatic colorectal cancer patients. Br J Cancer. 2010;102:151–61.
Zhang Y, Yang X, Wang R. Zhang X Prognostic value of PDZ-binding kinase/T-LAK cell-originated protein Kinase (PBK/TOPK) in patients with cancer. J Cancer. 2019;10:131–7.
Park JH, Nishidate T, Nakamura Y. Katagiri T Critical roles of T-LAK cell-originated protein kinase in cytokinesis. Cancer Sci. 2010;101:403–11.
Zou J, Kuang W, Hu J. Rao H miR-216b promotes cell growth and enhances chemosensitivity of colorectal cancer by suppressing PDZ-binding kinase. Biochem Biophys Res Commun. 2017;488:247–52.
Pirovano G, Ashton TM, Herbert KJ, Bryant RJ, Verrill CL, Cerundolo L, et al. TOPK modulates tumour-specific radiosensitivity and correlates with recurrence after prostate radiotherapy. Br J Cancer. 2017;117:503–12.
Shats I, Gatza ML, Chang JT, Mori S, Wang J, Rich J, et al. Using a stem cell-based signature to guide therapeutic selection in cancer. Cancer Res. 2011;71:1772–80.
Matsumoto S, Abe Y, Fujibuchi T, Takeuchi T, Kito K, Ueda N, et al. Characterization of a MAPKK-like protein kinase TOPK. Biochem Biophys Res Commun. 2004;325:997–1004.
Herbert KJ, Ashton TM, Prevo R, Pirovano G. Higgins GS T-LAK cell-originated protein kinase (TOPK): an emerging target for cancer-specific therapeutics. Cell Death Dis. 2018;9:1089.
Kim DJ, Li Y, Reddy K, Lee MH, Kim MO, Cho YY, et al. Novel TOPK inhibitor HI-TOPK-032 effectively suppresses colon cancer growth. Cancer Res. 2012;72:3060–8.
Matsuo Y, Park JH, Miyamoto T, Yamamoto S, Hisada S, Alachkar H, et al. TOPK inhibitor induces complete tumor regression in xenograft models of human cancer through inhibition of cytokinesis. Sci Transl Med. 2014;6:259ra145.
Gao G, Zhang T, Wang Q, Reddy K, Chen H, Yao K, et al. ADA-07 Suppresses solar ultraviolet-induced skin carcinogenesis by directly inhibiting TOPK. Mol Cancer Ther. 2017;16:1843–54.
Zeng X, Liu L, Zheng M, Sun H, Xiao J, Lu T, et al. Pantoprazole, an FDA-approved proton-pump inhibitor, suppresses colorectal cancer growth by targeting T-cell-originated protein kinase. Oncotarget. 2016;7:22460–73.
Zheng M, Luan S, Gao S, Cheng L, Hao B, Li J, et al. Proton pump inhibitor ilaprazole suppresses cancer growth by targeting T-cell-originated protein kinase. Oncotarget. 2017;8:39143–53.
Zhao R, Huang H, Choi BY, Liu X, Zhang M, Zhou S, et al. Cell growth inhibition by 3-deoxysappanchalcone is mediated by directly targeting the TOPK signaling pathway in colon cancer. Phytomedicine. 2019;61:152813.
Gao T, Hu Q, Hu X, Lei Q, Feng Z, Yu X, et al. Novel selective TOPK inhibitor SKLB-C05 inhibits colorectal carcinoma growth and metastasis. Cancer Lett. 2019;445:11–23.
Hu Q-F, Gao T-T, Shi Y-J, Lei Q, Liu Z-H, Feng Q, et al. Design, synthesis and biological evaluation of novel 1-phenyl phenanthridin-6(5H)-one derivatives as anti-tumor agents targeting TOPK. Eur J Med Chem. 2019;162:407–22.
Gilabert-Oriol R, Sutherland BW, Anantha M, Pallaoro A. Bally MB Liposomal OTS964, a TOPK inhibitor: a simple method to estimate OTS964 association with liposomes that relies on enhanced OTS964 fluorescence when bound to albumin. Drug Deliv Transl Res. 2019.
Pirovano G, Roberts S, Brand C, Donabedian PL, Mason C, de Souza PD, et al. [18F]FE-OTS964: a small molecule targeting TOPK for in vivo PET imaging in a glioblastoma xenograft model. Mol Imaging Biol. 2019;21:705–12.
Lin A, Giuliano CJ, Palladino A, John KM, Abramowicz C, Yuan ML, et al. Off-target toxicity is a common mechanism of action of cancer drugs undergoing clinical trials. Sci Transl Med. 2019;11.
Bem M, Badea F, Draghici C, Caproiu M, Vasilescu M, Voicescu M, et al. Synthesis and fluorescent properties of new derivatives of 4-amino-7-nitrobenzofurazan. Arkivoc. 2007;2007.
Feroldi F, Verlaan M, Knaus H, Davidoiu V, Vugts DJ, van Dongen GAMS, et al. High resolution combined molecular and structural optical imaging of colorectal cancer in a xenograft mouse model. Biomed Opt Express. 2018;9:6186–204.
Kuipers EJ, Grady WM, Lieberman D, Seufferlein T, Sung JJ, Boelens PG, et al. Colorectal cancer. Nat Rev Dis Primers. 2015;1:15065.
Joshi BP. Wang TD Targeted optical imaging agents in cancer: focus on clinical applications. Contrast Media Mol Imaging. 2018;2018:2015237.
Cheng Y. Prusoff WH Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–108.
Acknowledgments
The authors wish to acknowledge the support of Memorial Sloan Kettering Cancer Center’s Small Animal Imaging Core Facility, Radiochemistry & Molecular Imaging Probes Core Facility, Integrated Genomics Core Facility, Nuclear Magnetic Resonance Core Facility, Media Preparation Core Facility, and Molecular Cytology Core Facility. We wish to thank Dr. Pat Zanzonico and Ms. Valerie Longo for technical support with IVIS imaging.
Funding
This work was supported by National Institutes of Health grants R01 CA204441 (T.R.), P30 CA008748, and the Memorial Sloan Kettering Imaging and Radiation Sciences Program (T.R.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
T.R. is shareholder of Summit Biomedical Imaging, LLC and paid consultant for Theragnostics, Inc. G.P. and S.R. declare no conflict of interest.
Ethical approval (animal work)
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Preclinical Imaging
Electronic supplementary material
ESM 1
(PDF 3165 kb)
Rights and permissions
About this article
Cite this article
Pirovano, G., Roberts, S. & Reiner, T. TOPKi-NBD: a fluorescent small molecule for tumor imaging. Eur J Nucl Med Mol Imaging 47, 1003–1010 (2020). https://doi.org/10.1007/s00259-019-04608-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-019-04608-w