Abstract
Purpose
Given the challenges posed by the clinical diagnosis of atypical Alzheimer’s disease (AD) variants and the limited imaging evidence available in the prodromal phases of atypical AD, we assessed brain hypometabolism patterns at the single-subject level in the AD variants spectrum. Specifically, we tested the accuracy of [18F]FDG-PET brain hypometabolism, as a biomarker of neurodegeneration, in supporting the differential diagnosis of atypical AD variants in individuals with dementia and mild cognitive impairment (MCI).
Methods
We retrospectively collected N = 67 patients with a diagnosis of typical AD and AD variants according to the IWG-2 criteria (22 typical-AD, 15 frontal variant-AD, 14 logopenic variant-AD and 16 posterior variant-AD). Further, we included N = 11 MCI subjects, who subsequently received a clinical diagnosis of atypical AD dementia at follow-up (21 ± 11 months). We assessed brain hypometabolism patterns at group- and single-subject level, using W-score maps, measuring their accuracy in supporting differential diagnosis. In addition, the regional prevalence of cerebral hypometabolism was computed to identify the most vulnerable core regions.
Results
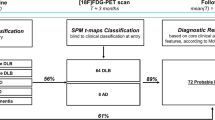
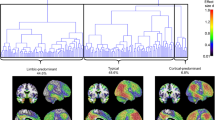
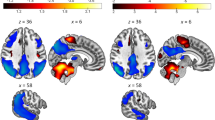
W-score maps pointed at distinct, specific patterns of hypometabolism in typical and atypical AD variants, confirmed by the assessment of core hypometabolism regions, showing that each variant was characterized by specific regional vulnerabilities, namely in occipital, left-sided, or frontal brain regions. ROC curves allowed discrimination among AD variants and also non-AD dementia (i.e., dementia with Lewy bodies and behavioral variant of frontotemporal dementia), with high sensitivity and specificity. Notably, we provide preliminary evidence that, even in AD prodromal phases, these specific [18F]FDG-PET patterns are already detectable and predictive of clinical progression to atypical AD variants at follow-up.
Conclusions
The AD variant-specific patterns of brain hypometabolism, highly consistent at single-subject level and already evident in the prodromal stages, represent relevant markers of disease neurodegeneration, with highly supportive diagnostic and prognostic role.





Similar content being viewed by others
References
Warren JD, Fletcher PD, Golden HL. The paradox of syndromic diversity in Alzheimer disease. Nat Rev Neurol. 2012;8:451–64.
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–44.
Crutch SJ, Schott JM, Rabinovici GD, Murray M, Snowden JS, Van Der Flier WM, et al. Consensus classification of posterior cortical atrophy. Alzheimers Dement. 2017;13:870–84.
Crutch SJ, Lehmann M, Schott JM, Rabinovici GD, Rossor MN, Fox NC. Posterior cortical atrophy. Lancet Neurol. 2012;11:170–8.
Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;11:1006–14.
Teichmann M, Kas A, Boutet C, Ferrieux S, Nogues M, Samri D, et al. Deciphering logopenic primary progressive aphasia: a clinical, imaging and biomarker investigation. Brain. 2013;136:3474–88.
Ossenkoppele R, Pijnenburg YAL, Perry DC, Cohn-Sheehy BI, Scheltens NME, Vogel JW, et al. The behavioural/dysexecutive variant of Alzheimer’s disease: clinical, neuroimaging and pathological features. Brain. 2015;138:2732–49.
Taylor KI, Probst A, Miserez AR, Monsch AU, Tolnay M. Clinical course of neuropathologically confirmed frontal-variant Alzheimer’s disease. Nat Clin Pract Neurol. 2008;4:226–32.
Whitwell JL, Graff-radford J, Tosakulwong N, Weigand SD, Machulda MM, Senjem ML, et al. Imaging correlations of tau, amyloid, metabolism, and atrophy in typical and atypical Alzheimer’s disease. Alzheimers Dement. 2018;14:1005–14.
McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–9.
Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, et al. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol. 2014;13:614–29.
Koedam ELGE, Lauffer V, Van Der Vlies AE, Van Der Flier WM, Scheltens P, Pijnenburg YAL. Early-versus late-onset Alzheimer’s disease: more than age alone. J Alzheimers Dis. 2010;19:1401–8.
Lukic AS, Andrews RD, Bourakova V, Rabinovici GD, Matthews DC. MRI, FDG and early frame amyloid image classifiers to characterize and differentiate Alzheimer’s disease variants and non-AD dementias. Alzheimers Dement. 2018;14:1429–30.
Jack CR, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14:535–62.
Stoessl AJ. Glucose utilization: still in the synapse. Nat Neurosci. 2017;20:382–4.
Perani D. FDG-PET and amyloid-PET imaging: the diverging paths. Curr Opin Neurol. 2014;27:405–13.
Taswell C, Villemagne VL, Yates P, Shimada H, Leyton CE, Ballard KJ, et al. 18F-FDG PET improves diagnosis in patients with focal-onset dementias. J Nucl Med. 2015;56:1547–53.
Iaccarino L, Sala A, Caminiti SP, Perani D. The emerging role of PET imaging in dementia. F1000Research. 2017;6:1830.
Caminiti SP, Sala A, Iaccarino L, Beretta L, Pilotto A, Gianolli L, et al. Brain glucose metabolism in Lewy body dementia : implications for diagnostic criteria. Alzheimers Res Ther. 2019;11:20.
Caminiti SP, Ballarini T, Sala A, Cerami C, Presotto L, Santangelo R, et al. FDG-PET and CSF biomarker accuracy in prediction of conversion to different dementias in a large multicentre MCI cohort. NeuroImage Clin. 2018;28:167–77.
Cerami C, Della Rosa PA, Magnani G, Santangelo R, Marcone A, Cappa SF, et al. Brain metabolic maps in mild cognitive impairment predict heterogeneity of progression to dementia. NeuroImage Clin. 2014;5:187–94.
Sörensen A, Blazhenets G, Rücker G, Schiller F, Meyer PT, Frings L. Prognosis of conversion of mild cognitive impairment to Alzheimer’ s dementia by voxel-wise cox regression based on FDG PET data. NeuroImage Clin. 2019;21:101637.
Nestor PJ, Altomare D, Festari C, Drzezga A, Rivolta J, Walker Z, et al. Clinical utility of FDG-PET for the differential diagnosis among the main forms of dementia. Eur J Nucl Med Mol Imaging. 2018;45:1509–25.
La Joie R, Perrotin A, Barre L, Hommet C, Mezenge F, Ibazizene M, et al. Region-specific hierarchy between atrophy, hypometabolism, and β-amyloid (Aβ) load in Alzheimer’s disease dementia. J Neurosci. 2012;32:16265–73.
Sjogren M, Vanderstichele H, Hans Å, Zachrisson O, Edsbagge M, Wikkelsø C, et al. Tau and Ab42 in cerebrospinal fluid from healthy adults 21–93 years of age : establishment of reference values. Clin Chem. 2001;47:1776–81.
Petersen RC, Roberts RO, Knopman DS, Boeve BF, Geda YE, Ivnik RJ, et al. Mild cognitive impairment ten years later. Arch Neurol. 2009;66:1447–55.
Borruat FX. Posterior cortical atrophy : review of the recent literature. Curr Neurol Neurosci Rep. 2013;13:406.
Sabbagh MN, Schäuble B, Anand K, Richards D, Murayama S, Akatsu H, et al. Histopathology and florbetaben PET in patients incorrectly diagnosed with Alzheimer’s disease. J Alzheimers Dis. 2017;56:441–6.
McKeith IG, Dickson DW, Lowe J, Emre M, Brien JTO, Feldman H, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB consortium. Neurology. 2005;65:1863–72.
Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–77.
Suárez-González A, Crutch SJ, Franco-Macias E, Gil-Néciga E. Neuropsychiatric symptoms in posterior cortical atrophy and Alzheimer disease. J Geriatr Psychiatry Neurol. 2016;29:65–71.
Josephs KA, Whitwell JL, Boeve BF, Knopman DS, Tang-Wai DF, Drubach DA, et al. Visual hallucinations in posterior cortical atrophy. Arch Neurol. 2006;63:1427–32.
Riedl V, Bienkowska K, Strobel C, Tahmasian M, Grimmer T, Friston KJ, et al. Local activity determines functional connectivity in the resting human brain : a simultaneous FDG-PET / fMRI study. J Neurosci. 2014;34:6260–6.
Della Rosa PA, Cerami C, Gallivanone F, Prestia A, Caroli A, Castiglioni I, et al. A standardized [18F]-FDG-PET template for spatial normalization in statistical parametric mapping of dementia. Neuroinformatics. 2014;12:575–93.
Buchert R, Wilke F, Chakrabarti B, Martin B, Brenner W, Mester J, et al. Adjusted scaling of FDG positron emission tomography images for statistical evaluation in patients with suspected Alzheimer’s disease. J Neuroimaging. 2005;15:348–55.
Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89.
Phillips JS, Da Re F, Dratch L, Xie SX, Irwin DJ, McMillan CT, et al. Neocortical origin and progression of gray matter atrophy in nonamnestic Alzheimer’s disease. Neurobiol Aging. 2018;63:75–87.
Phillips JS, Das SR, McMillan CT, Irwin DJ, Roll EE, Da Re F, et al. Tau PET imaging predicts cognition in atypical variants of Alzheimer’s disease. Hum Brain Mapp. 2018;39:691–708.
Perani D, Cerami C, Caminiti SP, Santangelo R, Coppi E, Ferrari L, et al. Cross-validation of biomarkers for the early differential diagnosis and prognosis of dementia in a clinical setting. Eur J Nucl Med Mol Imaging. 2016;43:499–508.
Iaccarino L, Chiotis K, Alongi P, Almkvist O, Wall A, Cerami C, et al. A cross-validation of FDG- and amyloid-PET biomarkers in mild cognitive impairment for the risk prediction to dementia due to Alzheimer’s disease in a clinical setting. J Alzheimers Dis. 2017;59:603–14.
Cerami C, Dodich A, Greco L, Iannaccone S, Magnani G, Marcone A, et al. The role of single-subject brain metabolic patterns in the early differential diagnosis of primary progressive aphasias and in prediction of progression to dementia. J Alzheimers Dis. 2017;55:183–97.
Cerami C, Crespi C, Della Rosa PA, Dodich A, Marcone A, Magnani G, et al. Brain changes within the visuo-spatial attentional network in posterior cortical atrophy. J Alzheimers Dis. 2015;43:385–95.
Smailagic N, Lafortune L, Kelly S, Hyde C, Brayne C. 18F-FDG PET for prediction of conversion to Alzheimer’s disease dementia in people with mild cognitive impairment: an updated systematic review of test accuracy. J Alzheimers Dis. 2018.
Perani D, Della Rosa PA, Cerami C, Gallivanone F, Fallanca F, Vanoli EG, et al. Validation of an optimized SPM procedure for FDG-PET in dementia diagnosis in a clinical setting. NeuroImage Clin. 2014;6:445–54.
Caminiti SP, Alongi P, Majno L, Volontè MA, Cerami C, Gianolli L, et al. Evaluation of an optimized [18F] fluoro-deoxy-glucose positron emission tomography voxel-wise method to early support differential diagnosis in atypical Parkinsonian disorders. Eur J Neurol. 2017;24:687–e26.
Iaccarino L, Sala A, Perani D. Predicting long-term clinical stability in amyloid-positive subjects by FDG-PET. Ann Clin Transl Neurol. 2019;6:1113–20.
Cerami C, Della Rosa PA, Magnani G, Santangelo R, Marcone A, Cappa SF, et al. Brain metabolic maps in mild cognitive impairment predict heterogeneity of progression to dementia. NeuroImage Clin. 2015;7:187–94.
Drzezga A, Lautenschlager N, Siebner H, Riemenschneider M, Willoch F, Minoshima S, et al. Cerebral metabolic changes accompanying conversion of mild cognitive impairment into Alzheimer’s disease: a PET follow-up study. Eur J Nucl Med Mol Imaging. 2003;30:1104–13.
Mosconi L, Mistur R, Switalski R, Tsui WH, Glodzik L, Li Y, et al. FDG-PET changes in brain glucose metabolism from normal cognition to pathologically verified Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2009;36:811–22.
Nestor PJ, Caine D, Fryer TD, Clarke J, Hodges JR. The topography of metabolic deficits in posterior cortical atrophy (the visual variant of Alzheimer’s disease) with FDG-PET. J Neurol Neurosurg Psychiatry. 2003;74:1521–9.
Wilson SM, Henry ML, Besbris M, Ogar JM, Dronkers NF, Jarrold W, et al. Connected speech production in three variants of primary progressive aphasia. Brain. 2010;133:2069–88.
Rogalski E, Cobia D, Harrison TM, Wieneke C, Thompson CK, Weintraub S, et al. Anatomy of language impairments in primary progressive aphasia. J Neurosci. 2011;31:3344–50.
Madhavan A, Whitwell JL, Weigand SD, Duffy JR, Strand EA, Machulda MM, et al. FDG PET and MRI in Logopenic primary progressive aphasia versus dementia of the Alzheimer’s type. PLoS One. 2013;8:e62471.
Rabinovici GD, Jagust WJ, Furst AJ, Ogar JM, Racine CA, Mormino EC, et al. Aβ amyloid and glucose metabolism in three variants of primary progressive aphasia. Ann Neurol. 2008;64:388–401.
Matias-Guiu JA, Cabrera-Martín MN, Moreno-Ramos T, García-Ramos R, Porta-Etessam J, Carreras JL, et al. Clinical course of primary progressive aphasia: clinical and FDG-PET patterns. J Neurol. 2015;262:570–7.
Rogalski E, Sridhar J, Rader B, Martersteck A, Chen K, Cobia D, et al. Aphasic variant of Alzheimer disease: clinical, anatomic, and genetic features. Neurology. 2016;87:1337–43.
Sajjadi SA, Sheikh-Bahaei N, Cross J, Gillard JH, Scoffings D, Nestor PJ. Can MRI visual assessment differentiate the variants of primary-progressive aphasia? Am J Neuroradiol. 2017;38:954–60.
Dronse J, Fliessbach K, Bischof GN, Von Reutern B, Faber J, Hammes J, et al. In vivo patterns of tau pathology, amyloid-β burden, and neuronal dysfunction in clinical variants of Alzheimer’s disease. J Alzheimers Dis. 2017;55:465–71.
Woodward MC, Rowe CC, Jones G, Villemagne VL, Varos TA. Differentiating the frontal presentation of Alzheimer’s disease with FDG-PET. J Alzheimers Dis. 2015;44:233–42.
Dickerson BC, Wolk DA. Dysexecutive versus amnesic phenotypes of very mild Alzheimer’s disease are associated with distinct clinical, genetic and cortical thinning characteristics. J Neurol Neurosurg Psychiatry. 2011;82:45–51.
Lehmann M, Ghosh PM, Madison C, Laforce R, Corbetta-Rastelli C, Weiner MW, et al. Diverging patterns of amyloid deposition and hypometabolism in clinical variants of probable Alzheimer’s disease. Brain. 2013;136:844–58.
Hof PR, Vogt BA, Bouras C, Morrison JH. Atypical form of Alzheimer’s disease with prominent posterior cortical atrophy: a review of lesion distribution and circuit disconnection in cortical visual pathways. Vis Res. 1997;37:3609–25.
Johnson JK, Head E, Kim R, Starr A, Cotman CW. Clinical and pathological evidence for a frontal variant of Alzheimer disease. Arch Neurol. 1999;56:1233–9.
Mesulam MM, Weintraub S, Rogalski EJ, Wieneke C, Geula C, Bigio EH. Asymmetry and heterogeneity of Alzheimer’s and frontotemporal pathology in primary progressive aphasia. Brain. 2014;137:1176–92.
Silverman DHS, Gambhir SS, Huang HC, Schwimmer J, Kim S, Small GW, et al. Evaluating early dementia with and without assessment of regional cerebral metabolism by PET: a comparison of predicted costs and benefits. J Nucl Med. 2002;43:253–67.
Cerami C, Dodich A, Lettieri G, Cappa SF, Perani D. Different FDG-PET metabolic patterns at single-subject level in the behavioral variant of frontotemporal dementia. Cortex. 2016;83:101–12.
Teune LK, Bartels AL, De Jong BM, Willemsen ATM, Eshuis SA, De Vries JJ, et al. Typical cerebral metabolic patterns in neurodegenerative brain diseases. Mov Disord. 2010;25:2395–404.
Whitwell JL, Graff-Radford J, Singh TD, Drubach DA, Senjem ML, Spychalla AJ, et al. 18 F-FDG PET in posterior cortical atrophy and dementia with Lewy bodies. J Nucl Med. 2017;58:632–8.
Gupta V, Verma R, Ranjan R, Belho E, Mahajan H. Lewy body dementia and posterior cortical variant of Alzheimer’s disease: distinguishing imaging patterns based on 18F-FDG PET/CT and 99mTc-TRODAT SPECT scan. J Nucl Med. 2019;60:1491.
Nedelska Z, Ferman TJ, Boeve BF, Przybelski SA, Lesnick TG, Murray ME, et al. Pattern of brain atrophy rates in autopsy-confirmed dementia with Lewy bodies. Neurobiol Aging. 2015;36:452–61.
Middelkoop HAM, Van der Flier WM, Burton EJ, Lloyd AJ, Paling S, Barber R, et al. Dementia with Lewy bodies and AD are not associated with occipital lobe atrophy on MRI. Neurology. 2001;57:2117–20.
O’Donovan J, Watson R, Colloby SJ, Firbank MJ, Burton EJ, Barber R, et al. Does posterior cortical atrophy on MRI discriminate between Alzheimer’ s disease, dementia with Lewy bodies, and normal aging? Int Psychogeriatr. 2012;25:111–9.
Nordlund A, Rolstad S, Hellstro P, Sjo M, Hansen S, Wallin A. The Goteborg MCI study : mild cognitive impairment is a heterogeneous condition. J Neurol Neurosurg Psychiatry. 2005;76:1485–90.
Anchisi D, Borroni B, Franchesci M, Nasser K, Ferruccio F, Perani D. Heterogeneity of brain glucose metabolism in mild cognitive impairment and clinical progression to Alzheimer disease. Arch Neurol. 2005;62:1728–33.
Shaffer JL, Petrella JR, Sheldon FC, Choudhury KR, Calhoun VD, Coleman RE, et al. Predicting cognitive decline in subjects at risk for Alzheimer disease by using combined cerebrospinal fluid, MR imaging, and PET biomarkers. Radiology. 2013;266:583–91.
Jagust W. Imaging the evolution and pathophysiology of Alzheimer disease. Nat Rev Neurosci. 2018;19:687–700.
Kljajevic V, Jan M, Ewers M, Teipel S. Distinct pattern of hypometabolism and atrophy in preclinical and predementia Alzheimer’s disease. Neurobiol Aging. 2014;35:1973–81.
Raj A, Kuceyeski A, Weiner M. A network diffusion model of disease progression in dementia. Neuron. 2012;73:1204–15.
Sala A, Perani D. Brain molecular connectivity in neurodegenerative diseases: recent advances and new perspectives using Positron Emission Tomography. Front Neurosci. 2019;in press.
Funding
This study was funded by the Italian Ministry of Health (Ricerca Finalizzata Progetto Reti Nazionale AD NET-2011-02346784), “IVASCOMAR project “Identificazione, validazione e sviluppo commerciale di nuovi biomarcatori diagnostici prognostici per malattie complesse” (grant agreement no. CTN01_00177_165430)”.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by San Raffaele Hospital Ethical Committee.
Informed consent
Informed consent was obtained from all individual participants included in the study or their informed caregivers, as approved by San Raffaele Hospital Ethical Committee.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Neurology
Electronic supplementary material
ESM 1
(DOCX 641 kb)
Rights and permissions
About this article
Cite this article
Sala, A., Caprioglio, C., Santangelo, R. et al. Brain metabolic signatures across the Alzheimer’s disease spectrum. Eur J Nucl Med Mol Imaging 47, 256–269 (2020). https://doi.org/10.1007/s00259-019-04559-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-019-04559-2