Abstract
Purpose
To evaluate the safety and efficacy of 177Lu-DOTA-EB-TATE, a radiolabeled somatostatin analog modified by Evans blue, at escalating doses, was used to increase tumor retention in patients with progressive metastatic neuroendocrine tumors (NETs).
Methods
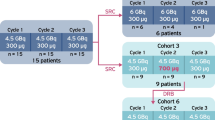
Thirty-three patients with metastatic NETs were prospectively enrolled into four groups: group A (n = 6, 43 ± 12 years) administered approximately 3.7 GBq (100 mCi) 177Lu-DOTATATE as controls; group B (n = 7, 55 ± 7 years) administered approximately 1.11 GBq (30 mCi) 177Lu-DOTA-EB-TATE; group C (n = 6, 55 ± 10 years) administered approximately 1.85 GBq (50 mCi) 177Lu-DOTA-EB-TATE; group D (n = 14, 50 ± 10 years) administered approximately 3.7 GBq (100 mCi) 177Lu-DOTA-EB-TATE. Treatment-related adverse events were graded according to the CTCAE v.5.0. 68Ga-DOTATATE PET/CT were performed at baseline and 2–3 months after treatment for response evaluation.
Results
Administration was well tolerated. No CTC 3/4 hematotoxicity, nephrotoxicity, or hepatotoxicity was observed during or after treatment in groups A–C. In group D, CTC-3 hematotoxicity was recorded in 2 patients with multicourse chemotherapy previously. After one-cycle treatment, the SUVmax decreased in group C (Δ% = − 17.4 ± 29.3%) and group D (Δ% = − 15.1 ± 39.1%), but greatly increased in group B (Δ% = 30.0 ± 68.0%) and mildly increased in group A (Δ% = 5.4 ± 45.9%). Referring to EORTC criteria, 16.7% (1/6), 0% (0/7), 50% (3/6), and 50% (7/14) were evaluated as partial response in groups A, B, C, and D, respectively. When selecting lesions with comparable baseline SUVmax ranging from 15 to 40, SUVmax showed no significant decrease in group B (Δ% = − 7.3 ± 24.5%) (P = 0.214), significant decrease in group C (Δ% = − 34.9 ± 12.4%) (P = 0.001), and in group D (Δ% = − 17.9 ± 19.7%) (P = 0.012) as compared with group A with increased SUVmax (Δ% = 8.4 ± 48.8%). SUVmax significantly decreased in the EBTATE groups (groups B–D combined) (Δ% = − 19.0 ± 21.5%) as compared with the TATE group (P = 0.045).
Conclusion
177Lu-DOTA-EB-TATE is well tolerated and is more effective than 177Lu-DOTATATE. Both 1.85 GBq (50 mCi) and 3.7 GBq (100 mCi) doses appear to be more effective than 1.11 GBq (30 mCi) dose. Further investigation with more cycles of 177Lu-DOTA-EB-TATE treatment and longer follow-up is warranted.
Trial registration
Treatment Using 177Lu-DOTA-EB-TATE in Patients with Advanced Neuroendocrine Tumors (NCT03478358). URL: https://register.clinicaltrials.gov/prs/app/action/ViewOrUnrelease?uid=U0001JRW&ts=13&sid=S0007RNX&cx=y3yqv4






Similar content being viewed by others
References
Modlin IM, Oberg K, Chung DC, Jensen RT, de Herder WW, Thakker RV, et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 2008;9:61–72. https://doi.org/10.1016/S1470-2045(07)70410-2.
Werner RA, Weich A, Kircher M, Solnes LB, Javadi MS, Higuchi T, et al. The theranostic promise for neuroendocrine tumors in the late 2010s - where do we stand, where do we go? Theranostics. 2018;8:6088–100. https://doi.org/10.7150/thno.30357.
Ito T, Sasano H, Tanaka M, Osamura RY, Sasaki I, Kimura W, et al. Epidemiological study of gastroenteropancreatic neuroendocrine tumors in Japan. J Gastroenterol. 2010;45:234–43. https://doi.org/10.1007/s00535-009-0194-8.
Luke C, Price T, Townsend A, Karapetis C, Kotasek D, Singhal N, et al. Epidemiology of neuroendocrine cancers in an Australian population. Cancer Causes Control. 2010;21:931–8. https://doi.org/10.1007/s10552-010-9519-4.
Paganelli G, Zoboli S, Cremonesi M, Bodei L, Ferrari M, Grana C, et al. Receptor-mediated radiotherapy with 90Y-DOTA-D-Phe1-Tyr3-octreotide. Eur J Nucl Med. 2001;28:426–34.
McCarthy KE, Woltering EA, Espenan GD, Cronin M, Maloney TJ, Anthony LB. In situ radiotherapy with 111In-pentetreotide: initial observations and future directions. Cancer J Sci Am. 1998;4:94–102.
Valkema R, De Jong M, Bakker WH, Breeman WA, Kooij PP, Lugtenburg PJ, et al. Phase I study of peptide receptor radionuclide therapy with [In-DTPA]octreotide: the Rotterdam experience. Semin Nucl Med. 2002;32:110–22.
Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, et al. Phase 3 trial of 177Lu-Dotatate for midgut neuroendocrine tumors. N Engl J Med. 2017;376:125–35. https://doi.org/10.1056/NEJMoa1607427.
Tian R, Jacobson O, Niu G, Kiesewetter DO, Wang Z, Zhu G, et al. Evans blue attachment enhances somatostatin receptor subtype-2 imaging and radiotherapy. Theranostics. 2018;8:735–45. https://doi.org/10.7150/thno.23491.
Zhang J, Wang H, Jacobson Weiss O, Cheng Y, Niu G, Li F, et al. Safety, pharmacokinetics and dosimetry of a long-acting radiolabeled somatostatin analogue 177Lu-DOTA-EB-TATE in patients with advanced metastatic neuroendocrine tumors. J Nucl Med. 2018;59:1699–705. https://doi.org/10.2967/jnumed.118.209841.
Kwekkeboom DJ, de Herder WW, Kam BL, van Eijck CH, van Essen M, Kooij PP, et al. Treatment with the radiolabeled somatostatin analog [177Lu-DOTA0,Tyr3]octreotate: toxicity, efficacy, and survival. J Clin Oncol. 2008;26:2124–30. https://doi.org/10.1200/JCO.2007.15.2553.
Cives M, Strosberg J. Radionuclide therapy for neuroendocrine tumors. Curr Oncol Rep. 2017;19:9. https://doi.org/10.1007/s11912-017-0567-8.
Del Prete M, Buteau FA, Beauregard JM. Personalized 177Lu-octreotate peptide receptor radionuclide therapy of neuroendocrine tumours: a simulation study. Eur J Nucl Med Mol Imaging. 2017;44:1490–500. https://doi.org/10.1007/s00259-017-3688-2.
Ehlerding EB, Lan X, Cai W. “Albumin Hitchhiking” with an Evans blue analog for cancer theranostics. Theranostics. 2018;8:812–4. https://doi.org/10.7150/thno.24183.
Liu Z, Chen X. Simple bioconjugate chemistry serves great clinical advances: albumin as a versatile platform for diagnosis and precision therapy. Chem Soc Rev. 2016;45:1432–56. https://doi.org/10.1039/c5cs00158g.
Evans HM, Schulemann W. The action of vital stains belonging to the Benzidine group. Science. 1914;39:443–54. https://doi.org/10.1126/science.39.1004.443.
Freedman FB, Johnson JA. Equilibrium and kinetic properties of the Evans blue-albumin system. Am J Phys. 1969;216:675–81. https://doi.org/10.1152/ajplegacy.1969.216.3.675.
Lau J, Jacobson O, Niu G, Lin KS, Benard F, Chen X. Bench to bedside: albumin binders for improved cancer radioligand therapies. Bioconjug Chem. 2019;30:487–502. https://doi.org/10.1021/acs.bioconjchem.8b00919.
Bandara N, Jacobson O, Mpoy C, Chen X, Rogers BE. Novel structural modification based on Evans blue dye to improve pharmacokinetics of a somastostatin-receptor-based theranostic agent. Bioconjug Chem. 2018;29:2448–54. https://doi.org/10.1021/acs.bioconjchem.8b00341.
Rolleman EJ, Kooij PP, de Herder WW, Valkema R, Krenning EP, de Jong M. Somatostatin receptor subtype 2-mediated uptake of radiolabelled somatostatin analogues in the human kidney. Eur J Nucl Med Mol Imaging. 2007;34:1854–60. https://doi.org/10.1007/s00259-007-0457-7.
Forrer F, Krenning EP, Kooij PP, Bernard BF, Konijnenberg M, Bakker WH, et al. Bone marrow dosimetry in peptide receptor radionuclide therapy with [177Lu-DOTA(0),Tyr(3)]octreotate. Eur J Nucl Med Mol Imaging. 2009;36:1138–46. https://doi.org/10.1007/s00259-009-1072-6.
Bodei L, Kidd M, Paganelli G, Grana CM, Drozdov I, Cremonesi M, et al. Long-term tolerability of PRRT in 807 patients with neuroendocrine tumours: the value and limitations of clinical factors. Eur J Nucl Med Mol Imaging. 2015;42:5–19. https://doi.org/10.1007/s00259-014-2893-5.
Paganelli G, Sansovini M, Ambrosetti A, Severi S, Monti M, Scarpi E, et al. 177 Lu-Dota-octreotate radionuclide therapy of advanced gastrointestinal neuroendocrine tumors: results from a phase II study. Eur J Nucl Med Mol Imaging. 2014;41:1845–51. https://doi.org/10.1007/s00259-014-2735-5.
Bergsma H, Konijnenberg MW, Kam BL, Teunissen JJ, Kooij PP, de Herder WW, et al. Subacute haematotoxicity after PRRT with (177)Lu-DOTA-octreotate: prognostic factors, incidence and course. Eur J Nucl Med Mol Imaging. 2016;43:453–63. https://doi.org/10.1007/s00259-015-3193-4.
Sabet A, Ezziddin K, Pape UF, Ahmadzadehfar H, Mayer K, Poppel T, et al. Long-term hematotoxicity after peptide receptor radionuclide therapy with 177Lu-octreotate. J Nucl Med. 2013;54:1857–61. https://doi.org/10.2967/jnumed.112.119347.
NETTER-1. Phase III in patients with midgut neuroendocrine tumors treated with 177Lu-DOTATATE: efficacy and safety results. Clin Adv Hematol Oncol. 2016;14:8–9.
Brieau B, Hentic O, Lebtahi R, Palazzo M, Ben Reguiga M, Rebours V, et al. High risk of myelodysplastic syndrome and acute myeloid leukemia after 177Lu-octreotate PRRT in NET patients heavily pretreated with alkylating chemotherapy. Endocr Relat Cancer. 2016;23:L17–23. https://doi.org/10.1530/ERC-15-0543.
Valkema R, Pauwels SA, Kvols LK, Kwekkeboom DJ, Jamar F, de Jong M, et al. Long-term follow-up of renal function after peptide receptor radiation therapy with (90)Y-DOTA(0),Tyr(3)-octreotide and (177)Lu-DOTA(0), Tyr(3)-octreotate. J Nucl Med. 2005;46(Suppl 1):83S–91S.
Kwekkeboom DJ, Teunissen JJ, Bakker WH, Kooij PP, de Herder WW, Feelders RA, et al. Radiolabeled somatostatin analog [177Lu-DOTA0,Tyr3] octreotate in patients with endocrine gastroenteropancreatic tumors. J Clin Oncol. 2005;23:2754–62. https://doi.org/10.1200/JCO.2005.08.066.
Forrer F, Uusijarvi H, Storch D, Maecke HR, Mueller-Brand J. Treatment with 177Lu-DOTATOC of patients with relapse of neuroendocrine tumors after treatment with 90Y-DOTATOC. J Nucl Med. 2005;46:1310–6.
Bergsma H, Konijnenberg MW, van der Zwan WA, Kam BL, Teunissen JJ, Kooij PP, et al. Nephrotoxicity after PRRT with (177)Lu-DOTA-octreotate. Eur J Nucl Med Mol Imaging. 2016;43:1802–11. https://doi.org/10.1007/s00259-016-3382-9.
Wang H, Cheng Y, Zhang J, Zang J, Li H, Liu Q, et al. Response to single low-dose (177)Lu-DOTA-EB-TATE treatment in patients with advanced neuroendocrine neoplasm: a prospective pilot study. Theranostics. 2018;8:3308–16. https://doi.org/10.7150/thno.25919.
Pencharz D, Walker M, Yalchin M, Quigley AM, Caplin M, Toumpanakis C, et al. Early efficacy of and toxicity from lutetium-177-DOTATATE treatment in patients with progressive metastatic NET. Nucl Med Commun. 2017;38:593–600. https://doi.org/10.1097/MNM.0000000000000685.
Funding
This study was supported by the Key Project on Inter-Governmental International Scientific and Technological Innovation Cooperation in National Key Projects of Research and Development Plan (2016YFE0115400), the Intramural Research Program (IRP), National Institute of Biomedical Imaging and Bioengineering (NIBIB), National Institutes of Health (NIH), and was also partly supported by the Chinese Academy of Medical Science Major Collaborative Innovation Project (2016-I2M-1-011), Capital Health Development Scientific Research Project (2018-1-4011), National Nature Science Foundation (81871392, 81741142, 3316 81701742), and Beijing Municipal Natural Science Foundation (7161012).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures involving human participants in studies were in accordance with the ethical standards of the Institute Review Board of Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, and with the principles of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Oncology – General
Rights and permissions
About this article
Cite this article
Liu, Q., Cheng, Y., Zang, J. et al. Dose escalation of an Evans blue–modified radiolabeled somatostatin analog 177Lu-DOTA-EB-TATE in the treatment of metastatic neuroendocrine tumors. Eur J Nucl Med Mol Imaging 47, 947–957 (2020). https://doi.org/10.1007/s00259-019-04530-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-019-04530-1