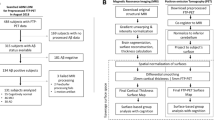
Abstract
Purpose
Amyloid (Aβ) brain deposition can occur in cognitively normal individuals and is associated with cortical volume abnormalities. Aβ-related volume changes are inconsistent across studies. Since volume is composed of surface area and thickness, the relative contribution of Aβ deposition on each of these metrics remains to be understood in cognitively normal individuals.
Methods
A group of 104 cognitively normal individuals underwent neuropsychological assessment, PiB-PET scan, and MRI acquisition. Surface-based cortical analyses were performed to investigate the effects of cortical and subcortical Aβ burden on cortical volume, thickness, and surface area. Mediation analyses were used to study the effect of thickness and surface area on Aβ-associated volume changes. We also investigated the relationships between structural metrics in clusters with abnormal morphology and regions underlying resting-state functional networks and cognitive performance.
Results
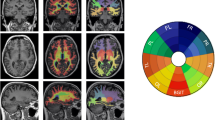
Cortical Aβ was not associated with cortical morphology. Subcortical Aβ burden was associated with changes in cortical volume, thickness, and surface area. Aβ-associated volume changes were driven by cortical surface area with or without thickness but never by thickness alone. Aβ-associated changes overlapped greatly with regions from the default mode network and were associated with lower performance in visuospatial abilities, episodic memory, and working memory.
Conclusions
In cognitively normal individuals, subcortical Aβ is associated with cortical volume, and this effect was driven by surface area with or without thickness. Aβ-associated cortical changes were found in the default mode network and affected cognitive performance. Our findings demonstrate the importance of studying subcortical Aβ and cortical surface area in normal ageing.



Similar content being viewed by others
References
Thal DR, Rub U, Orantes M, Braak H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58:1791–800.
Murray ME, Lowe VJ, Graff-Radford NR, Liesinger AM, Cannon A, Przybelski SA, et al. Clinicopathologic and 11C-Pittsburgh compound B implications of Thal amyloid phase across the Alzheimer’s disease spectrum. Brain. 2015;138:1370–81. https://doi.org/10.1093/brain/awv050.
Epelbaum S, Genthon R, Cavedo E, Habert MO, Lamari F, Gagliardi G, et al. Preclinical Alzheimer’s disease: a systematic review of the cohorts underlying the concept. Alzheimers Dement. 2017;13:454–67. https://doi.org/10.1016/j.jalz.2016.12.003.
Jack CR Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9:119–28. https://doi.org/10.1016/S1474-4422(09)70299-6.
Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–59.
Palmqvist S, Mattsson N, Hansson O. Alzheimer’s disease neuroimaging I. Cerebrospinal fluid analysis detects cerebral amyloid-beta accumulation earlier than positron emission tomography. Brain. 2016;139:1226–36. https://doi.org/10.1093/brain/aww015.
Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A. 2004;101:4637–42. https://doi.org/10.1073/pnas.0308627101.
Mormino EC, Smiljic A, Hayenga AO, Onami SH, Greicius MD, Rabinovici GD, et al. Relationships between beta-amyloid and functional connectivity in different components of the default mode network in aging. Cereb Cortex. 2011;21:2399–407. https://doi.org/10.1093/cercor/bhr025.
Sperling RA, Laviolette PS, O’Keefe K, O’Brien J, Rentz DM, Pihlajamaki M, et al. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron. 2009;63:178–88. https://doi.org/10.1016/j.neuron.2009.07.003.
de Flores R, La Joie R, Chetelat G. Structural imaging of hippocampal subfields in healthy aging and Alzheimer’s disease. Neuroscience. 2015;309:29–50. https://doi.org/10.1016/j.neuroscience.2015.08.033.
Storandt M, Mintun MA, Head D, Morris JC. Cognitive decline and brain volume loss as signatures of cerebral amyloid-beta peptide deposition identified with Pittsburgh compound B: cognitive decline associated with Abeta deposition. Arch Neurol. 2009;66:1476–81. https://doi.org/10.1001/archneurol.2009.272.
Fagan AM, Head D, Shah AR, Marcus D, Mintun M, Morris JC, et al. Decreased cerebrospinal fluid Abeta(42) correlates with brain atrophy in cognitively normal elderly. Ann Neurol. 2009;65:176–83. https://doi.org/10.1002/ana.21559.
Dore V, Villemagne VL, Bourgeat P, Fripp J, Acosta O, Chetelat G, et al. Cross-sectional and longitudinal analysis of the relationship between Abeta deposition, cortical thickness, and memory in cognitively unimpaired individuals and in Alzheimer disease. JAMA Neurol. 2013;70:903–11. https://doi.org/10.1001/jamaneurol.2013.1062.
Fjell AM, Walhovd KB, Fennema-Notestine C, McEvoy LK, Hagler DJ, Holland D, et al. Brain atrophy in healthy aging is related to CSF levels of Abeta1-42. Cereb Cortex. 2010;20:2069–79. https://doi.org/10.1093/cercor/bhp279.
Chetelat G, Villemagne VL, Pike KE, Baron JC, Bourgeat P, Jones G, et al. Larger temporal volume in elderly with high versus low beta-amyloid deposition. Brain. 2010;133:3349–58. https://doi.org/10.1093/brain/awq187.
Winkler AM, Greve DN, Bjuland KJ, Nichols TE, Sabuncu MR, Ha Berg AK, et al. Joint analysis of cortical area and thickness as a replacement for the analysis of the volume of the cerebral cortex. Cereb Cortex. 2018;28:738–49. https://doi.org/10.1093/cercor/bhx308.
Winkler AM, Kochunov P, Blangero J, Almasy L, Zilles K, Fox PT, et al. Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. Neuroimage. 2010;53:1135–46. https://doi.org/10.1016/j.neuroimage.2009.12.028.
Panizzon MS, Fennema-Notestine C, Eyler LT, Jernigan TL, Prom-Wormley E, Neale M, et al. Distinct genetic influences on cortical surface area and cortical thickness. Cereb Cortex. 2009;19:2728–35. https://doi.org/10.1093/cercor/bhp026.
Becker JA, Hedden T, Carmasin J, Maye J, Rentz DM, Putcha D, et al. Amyloid-beta associated cortical thinning in clinically normal elderly. Ann Neurol. 2011;69:1032–42. https://doi.org/10.1002/ana.22333.
Dickerson BC, Bakkour A, Salat DH, Feczko E, Pacheco J, Greve DN, et al. The cortical signature of Alzheimer’s disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb Cortex. 2009;19:497–510. https://doi.org/10.1093/cercor/bhn113.
Knopman DS, Lundt ES, Therneau TM, Vemuri P, Lowe VJ, Kantarci K, et al. Joint associations of beta-amyloidosis and cortical thickness with cognition. Neurobiol Aging. 2018;65:121–31. https://doi.org/10.1016/j.neurobiolaging.2018.01.017.
Whitwell JL, Tosakulwong N, Weigand SD, Senjem ML, Lowe VJ, Gunter JL, et al. Does amyloid deposition produce a specific atrophic signature in cognitively normal subjects? Neuroimage Clin. 2013;2:249–57. https://doi.org/10.1016/j.nicl.2013.01.006.
Dickerson BC, Feczko E, Augustinack JC, Pacheco J, Morris JC, Fischl B, et al. Differential effects of aging and Alzheimer’s disease on medial temporal lobe cortical thickness and surface area. Neurobiol Aging. 2009;30:432–40. https://doi.org/10.1016/j.neurobiolaging.2007.07.022.
Villeneuve S, Rabinovici GD, Cohn-Sheehy BI, Madison C, Ayakta N, Ghosh PM, et al. Existing Pittsburgh Compound-B positron emission tomography thresholds are too high: statistical and pathological evaluation. Brain. 2015;138:2020–33. https://doi.org/10.1093/brain/awv112.
Cho SH, Shin JH, Jang H, Park S, Kim HJ, Kim SE, et al. Amyloid involvement in subcortical regions predicts cognitive decline. Eur J Nucl Med Mol Imaging. 2018. doi:https://doi.org/10.1007/s00259-018-4081-5.
Hanseeuw BJ, Betensky RA, Mormino EC, Schultz AP, Sepulcre J, Becker JA, et al. PET staging of amyloidosis using striatum. Alzheimers Dement. 2018. doi:https://doi.org/10.1016/j.jalz.2018.04.011.
Hanseeuw BJ, Jonas V, Jackson J, Betensky RA, Rentz DM, Johnson KA, et al. Association of anxiety with subcortical amyloidosis in cognitively normal older adults. Mol Psychiatry. 2018. doi:https://doi.org/10.1038/s41380-018-0214-2.
Rahayel S, Bocti C, Sevigny Dupont P, Joannette M, Lavallee MM, Nikelski J, et al. Subcortical amyloid load is associated with shape and volume in cognitively normal individuals. Hum Brain Mapp. 2019. doi:https://doi.org/10.1002/hbm.24680.
Ad-Dab’bagh Y, Lyttelton O, Muehlboeck JS, Lepage C, Einarson D, Mok K, et al. The CIVET image-processing environment: a fully automated comprehensive pipeline for anatomical neuroimaging research. In: Corbetta M, editor. Proceedings of the 12th annual meeting of the organization for human brain mapping. 2006.
Nikelski J, Chertkow H, Evans A. Running with the beagle: a multi-modal, integrative imaging pipeline specialized for the processing of elderly brains. Human Amyloid Imaging Conference. 2012 p. 80.
Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–19. https://doi.org/10.1002/ana.20009.
Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89. https://doi.org/10.1006/nimg.2001.0978.
Reuter M, Schmansky NJ, Rosas HD, Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage. 2012;61:1402–18. https://doi.org/10.1016/j.neuroimage.2012.02.084.
Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–94. https://doi.org/10.1006/nimg.1998.0395.
Reuter M, Rosas HD, Fischl B. Highly accurate inverse consistent registration: a robust approach. Neuroimage. 2010;53:1181–96. https://doi.org/10.1016/j.neuroimage.2010.07.020.
Segonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, et al. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22:1060–75. https://doi.org/10.1016/j.neuroimage.2004.03.032.
Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–55.
Fischl B, Salat DH, van der Kouwe AJ, Makris N, Segonne F, Quinn BT, et al. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004;23(Suppl 1):S69–84. https://doi.org/10.1016/j.neuroimage.2004.07.016.
Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97. https://doi.org/10.1109/42.668698.
Fischl B, Liu A, Dale AM. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging. 2001;20:70–80. https://doi.org/10.1109/42.906426.
Segonne F, Pacheco J, Fischl B. Geometrically accurate topology-correction of cortical surfaces using nonseparating loops. IEEE Trans Med Imaging. 2007;26:518–29. https://doi.org/10.1109/TMI.2006.887364.
Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97:11050–5. https://doi.org/10.1073/pnas.200033797.
Dale AM, Sereno MI. Improved localizadon of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: a linear approach. J Cogn Neurosci. 1993;5:162–76. https://doi.org/10.1162/jocn.1993.5.2.162.
Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. https://doi.org/10.1006/nimg.1998.0396.
Fischl B, Sereno MI, Tootell RB, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp. 1999;8:272–84.
Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14:11–22.
Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–80. https://doi.org/10.1016/j.neuroimage.2006.01.021.
Rosas HD, Liu AK, Hersch S, Glessner M, Ferrante RJ, Salat DH, et al. Regional and progressive thinning of the cortical ribbon in Huntington’s disease. Neurology. 2002;58:695–701.
Kuperberg GR, Broome MR, McGuire PK, David AS, Eddy M, Ozawa F, et al. Regionally localized thinning of the cerebral cortex in schizophrenia. Arch Gen Psychiatry. 2003;60:878–88. https://doi.org/10.1001/archpsyc.60.9.878.
Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, et al. Thinning of the cerebral cortex in aging. Cereb Cortex. 2004;14:721–30. https://doi.org/10.1093/cercor/bhh032.
Im K, Lee JM, Lyttelton O, Kim SH, Evans AC, Kim SI. Brain size and cortical structure in the adult human brain. Cereb Cortex. 2008;18:2181–91. https://doi.org/10.1093/cercor/bhm244.
Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–65. https://doi.org/10.1152/jn.00338.2011.
Hayes AF. Introduction to mediation, moderation, and conditional process analysis: a regression-based approach. New York: The Guilford Press; 2013.
Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE. Permutation inference for the general linear model. Neuroimage. 2014;92:381–97. https://doi.org/10.1016/j.neuroimage.2014.01.060.
Williams J, Mackinnon DP. Resampling and distribution of the product methods for testing indirect effects in complex models. Struct Equ Modeling. 2008;15:23–51. https://doi.org/10.1080/10705510701758166.
Tosun D, Schuff N, Truran-Sacrey D, Shaw LM, Trojanowski JQ, Aisen P, et al. Relations between brain tissue loss, CSF biomarkers, and the ApoE genetic profile: a longitudinal MRI study. Neurobiol Aging. 2010;31:1340–54. https://doi.org/10.1016/j.neurobiolaging.2010.04.030.
Arenaza-Urquijo EM, Molinuevo JL, Sala-Llonch R, Sole-Padulles C, Balasa M, Bosch B, et al. Cognitive reserve proxies relate to gray matter loss in cognitively healthy elderly with abnormal cerebrospinal fluid amyloid-beta levels. J Alzheimers Dis. 2013;35:715–26. https://doi.org/10.3233/JAD-121906.
Raichle ME. The brain’s default mode network. Annu Rev Neurosci. 2015;38:433–47. https://doi.org/10.1146/annurev-neuro-071013-014030.
Dixon ML, De La Vega A, Mills C, Andrews-Hanna J, Spreng RN, Cole MW, et al. Heterogeneity within the frontoparietal control network and its relationship to the default and dorsal attention networks. Proc Natl Acad Sci U S A. 2018;115:E1598–E607. https://doi.org/10.1073/pnas.1715766115.
Nava E, Roder B. Adaptation and maladaptation insights from brain plasticity. Prog Brain Res. 2011;191:177–94. https://doi.org/10.1016/B978-0-444-53752-2.00005-9.
Beach TG, Sue LI, Walker DG, Sabbagh MN, Serrano G, Dugger BN, et al. Striatal amyloid plaque density predicts Braak neurofibrillary stage and clinicopathological Alzheimer’s disease: implications for amyloid imaging. J Alzheimers Dis. 2012;28:869–76. https://doi.org/10.3233/JAD-2011-111340.
Sepulcre J, Sabuncu MR, Becker A, Sperling R, Johnson KA. In vivo characterization of the early states of the amyloid-beta network. Brain. 2013;136:2239–52. https://doi.org/10.1093/brain/awt146.
Fortea J, Sala-Llonch R, Bartres-Faz D, Bosch B, Llado A, Bargallo N, et al. Increased cortical thickness and caudate volume precede atrophy in PSEN1 mutation carriers. J Alzheimers Dis. 2010;22:909–22. https://doi.org/10.3233/JAD-2010-100678.
Spreng RN, Sepulcre J, Turner GR, Stevens WD, Schacter DL. Intrinsic architecture underlying the relations among the default, dorsal attention, and frontoparietal control networks of the human brain. J Cogn Neurosci. 2013;25:74–86. https://doi.org/10.1162/jocn_a_00281.
Sestieri C, Shulman GL, Corbetta M. The contribution of the human posterior parietal cortex to episodic memory. Nat Rev Neurosci. 2017;18:183–92. https://doi.org/10.1038/nrn.2017.6.
Breukelaar IA, Antees C, Grieve SM, Foster SL, Gomes L, Williams LM, et al. Cognitive control network anatomy correlates with neurocognitive behavior: a longitudinal study. Hum Brain Mapp. 2017;38:631–43. https://doi.org/10.1002/hbm.23401.
Friederici AD, Gierhan SM. The language network. Curr Opin Neurobiol. 2013;23:250–4. https://doi.org/10.1016/j.conb.2012.10.002.
Kuznetsova KA, Maniega SM, Ritchie SJ, Cox SR, Storkey AJ, Starr JM, et al. Brain white matter structure and information processing speed in healthy older age. Brain Struct Funct. 2016;221:3223–35. https://doi.org/10.1007/s00429-015-1097-5.
Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62:42–52. https://doi.org/10.1016/j.neuron.2009.03.024.
Rakic P. Radial unit hypothesis of neocortical expansion. Novartis Found Symp. 2000;228:30–42 discussion −52.
Palaniyappan L, Liddle PF. Differential effects of surface area, gyrification and cortical thickness on voxel based morphometric deficits in schizophrenia. Neuroimage. 2012;60:693–9. https://doi.org/10.1016/j.neuroimage.2011.12.058.
Funding
This work was funded by the Canadian Institutes of Health Research (no. MOP123376) and the Institute of Aging (no. IA0120269). SJ was supported by a Chercheur boursier senior award from the Fonds de recherche du Québec—Santé (FRQ-S).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Christian Bocti declares investments at IMEKA. None of the other co-authors report having any conflicts of interests.
Ethical approval
All research protocols were reviewed and in accordance with ethical standards. They were approved by the Centre de recherche de l’Institut universitaire de gériatrie de Montréal, the Montreal Neurological Institute, and the Hospital Research Ethics Boards.
Informed consent
All subjects gave their informed consent prior to their participation in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Neurology
Electronic supplementary material
ESM 1
(DOCX 34.7 kb)
Rights and permissions
About this article
Cite this article
Rahayel, S., Bocti, C., Sévigny Dupont, P. et al. Subcortical amyloid relates to cortical morphology in cognitively normal individuals. Eur J Nucl Med Mol Imaging 46, 2358–2369 (2019). https://doi.org/10.1007/s00259-019-04446-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-019-04446-w