Abstract
Objective
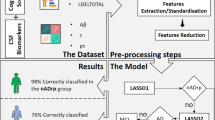
The pattern expression score (PES), i.e., the degree to which a pathology-related pattern is present, is frequently used in FDG-brain-PET analysis and has been shown to be a powerful predictor of conversion to Alzheimer’s disease (AD) in mild cognitive impairment (MCI). Since, inevitably, the PES is affected by non-pathological variability, our aim was to improve classification with the simple, yet novel approach to identify patterns of non-pathological variance in a separate control sample using principal component analysis and removing them from patient data (controls-based denoising, CODE) before calculating the PES.
Methods
Multi-center FDG-PET from 220 MCI patients (64 non-converter, follow-up ≥ 4 years; 156 AD converter, time-to-conversion ≤ 4 years) were obtained from the ADNI database. Patterns of non-pathological variance were determined from 262 healthy controls. An AD pattern was calculated from AD patients and controls. We predicted AD conversion based on PES only and on PES combined with neuropsychological features and ApoE4 genotype. We compared classification performance achieved with and without CODE and with a standard machine learning approach (support vector machine).
Results
Our model predicts that CODE improves the signal-to-noise ratio of AD-PES by a factor of 1.5. PES-based prediction of AD conversion improved from AUC 0.80 to 0.88 (p= 0.001, DeLong’s method), sensitivity 69 to 83%, specificity 81% to 88% and Matthews correlation coefficient (MCC) 0.45 to 0.66. Best classification (0.93 AUC) was obtained when combining the denoised PES with clinical features.
Conclusions
CODE, applied in its basic form, significantly improved prediction of conversion based on PES. The achieved classification performance was higher than with a standard machine learning algorithm, which was trained on patients, explainable by the fact that CODE used additional information (large sample of healthy controls). We conclude that the proposed, novel method is a powerful tool for improving medical image analysis that offers a wide spectrum of biomedical applications, even beyond image analysis.


Similar content being viewed by others
References
Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage 2007;38(1):95–113.
Bishop CM. Pattern recognition and machine learning (Information science and statistics). New York: Springer-Verlag; 2006.
Blazhenets G, Ma Y, Sorensen A, Rucker G, Schiller F, Eidelberg D, Frings L, Meyer PT. 2018. Principal component analysis of brain metabolism predicts development of Alzheimer’s dementia. J. Nucl. Med.
Caroli A, Prestia A, Chen K, Ayutyanont N, Landau SM, Madison CM, Haense C, Herholz K, Nobili F, Reiman EM, Jagust WJ, Frisoni GB, Perani D, Pupi A, Holthoff V, Salmon E, Baron JC, Drzezga A, Perneczky R, Didic M, Guedj E, Van Berckel BN, Ossenkoppele R, Morbelli S. Summary metrics to assess Alzheimer disease-related hypometabolic pattern with 18F-FDG PET: head-to-head comparison. J Nucl Med 2012;53(4):592–600.
De Carli F, Nobili F, Pagani M, Bauckneht M, Massa F, Grazzini M, Jonsson C, Peira E, Morbelli S, Arnaldi D. Accuracy and generalization capability of an automatic method for the detection of typical brain hypometabolism in prodromal Alzheimer disease. Eur J Nucl Med Mol Imaging 2019;46(2):334–347.
DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44(3):837–845.
Frings L, Hellwig S, Bormann T, Spehl TS, Buchert R, Meyer PT. Amyloid load but not regional glucose metabolism predicts conversion to Alzheimer’s dementia in a memory clinic population. Eur J Nucl Med Mol Imaging 2018;45(8):1442–1448.
Grimmer T, Wutz C, Alexopoulos P, Drzezga A, Forster S, Forstl H, Goldhardt O, Ortner M, Sorg C, Kurz A. Visual versus fully automated analyses of 18F-FDG and amyloid PET for prediction of dementia due to Alzheimer disease in mild cognitive impairment. J Nucl Med 2016;57(2):204–207.
Huang J, Lu J, Ling LCX. Comparing naive Bayes, decision trees, and SVM with AUC and accuracy. 3rd IEEE international conference on data mining, ICDM 2003, pp 553–556. IEEE Computer Society; 2003.
Jolliffe I. Principal component analysis. Berlin: Springer Verlag; 1986.
Li K, Chan W, Doody RS, Quinn J, Luo S. Prediction of conversion to Alzheimer’s disease with longitudinal measures and time-to-event data. J Alzheimers Dis 2017;58(2):361–371.
Ling CX, Huang J, Zhang H. AUC: a statistically consistent and more discriminating measure than accuracy. Proceedings of 18th international conference on Artificial Intelligence (IJCAI-2003), pp 519–524; 2003.
Liu K, Chen K, Yao L, Guo X. Prediction of mild cognitive impairment conversion using a combination of independent component analysis and the Cox model. Front Hum Neurosci 2017;11:33.
Martino ME, de Villoria JG, Lacalle-Aurioles M, Olazaran J, Cruz I, Navarro E, Garcia-Vazquez V, Carreras JL, Desco M. Comparison of different methods of spatial normalization of FDG-PET brain images in the voxel-wise analysis of MCI patients and controls. Ann Nucl Med 2013;27(7):600–609.
Morbelli S, Garibotto V, Van De Giessen E, Arbizu J, Chetelat G, Drezgza A, Hesse S, Lammertsma AA, Law I, Pappata’ S, Payoux P, Pagani M. A Cochrane review on brain [18F]FDG PET in dementia: limitations and future perspectives. Eur J Nucl Med Mol Imaging 2015;42(10):1487–1491.
Nobili F, Salmaso D, Morbelli S, Girtler N, Piccardo A, Brugnolo A, Dessi B, Larsson SA, Rodriguez G, Pagani M. Principal component analysis of FDG PET in amnestic MCI. Eur J Nucl Med Mol Imaging 2008;35(12):2191–2202.
Pagani M, Giuliani A, Oberg J, Chincarini A, Morbelli S, Brugnolo A, Arnaldi D, Picco A, Bauckneht M, Buschiazzo A, Sambuceti G, Nobili F. Predicting the transition from normal aging to Alzheimer’s disease:a statistical mechanistic evaluation of FDG-PET data. Neuroimage 2016;141:282–290.
Pagani M, Nobili F, Morbelli S, Arnaldi D, Giuliani A, Oberg J, Girtler N, Brugnolo A, Picco A, Bauckneht M, Piva R, Chincarini A, Sambuceti G, Jonsson C, De Carli F. Early identification of MCI converting to AD: a FDG PET study. Eur J Nucl Med Mol Imaging 2017;44(12):2042–2052.
Pearson K. Liii. on lines and planes of closest fit to systems of points in space. The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science 1901;2(11):559–572. https://doi.org/10.1080/14786440109462720.
Petersen RC, Aisen PS, Beckett LA, Donohue MC, Gamst AC, Harvey DJ, Jack CR, Jagust WJ, Shaw LM, Toga AW, Trojanowski JQ, Weiner MW. Alzheimer’s Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology 2010;74(3):201–209.
Shi L, Campbell G, Jones EA. The microArray quality control (MAQC)-II study of common practices for the development and validation of microarray-based predictive models. Nat Biotechnol 2010;28(8):827–838.
Smailagic N, Lafortune L, Kelly S, Hyde C, Brayne C. 18F-FDG PET for prediction of conversion to Alzheimer’s disease dementia in people with mild cognitive impairment: An updated systematic review of test accuracy. J Alzheimers Dis 2018;64(4):1175–1194.
Spetsieris PG, Eidelberg D. Scaled subprofile modeling of resting state imaging data in Parkinson’s disease: methodological issues. Neuroimage 2011;54(4):2899–2914.
Steiger JH. Tests for comparing elements of a correlation matrix. Psychol Bull 1980;87:245–251.
Sun X, Xu W. Fast implementation of DeLong’s algorithm for comparing the areas under correlated receiver operating characteristic curves. IEEE Signal Process Lett 2014;21(11):1389–1393.
Trzepacz PT, Yu P, Sun J, Schuh K, Case M, Witte MM, Hochstetler H, Hake A. Comparison of neuroimaging modalities for the prediction of conversion from mild cognitive impairment to Alzheimer’s dementia. Neurobiol Aging 2014;35(1):143–151.
Youden WJ. Index for rating diagnostic tests. Cancer 1950;3(1):32–35.
Acknowledgements
We acknowledge the funding received from the European Community’s Seventh Framework Programme (FP7/2007–2013) under grant agreement no. 603646 (MultISyn).
Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Funding
The research leading to these results received funding from the European Community’s Seventh Framework Programme (FP7/2007–2013) under grant agreement no. 603646 (MultISyn).
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
Dominik Blum declares that he has no conflict of interest. Inga Liepelt-Scarfone declares that she has no conflict of interest. Daniela Berg declares that she has no conflict of interest. Thomas Gasser declares that he has no conflict of interest. Christian la Fougère declares that he has no conflict of interest. Matthias Reimold declares that he has no conflict of interest.
Data used in preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Neurology
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Blum, D., Liepelt-Scarfone, I., Berg, D. et al. Controls-based denoising, a new approach for medical image analysis, improves prediction of conversion to Alzheimer’s disease with FDG-PET. Eur J Nucl Med Mol Imaging 46, 2370–2379 (2019). https://doi.org/10.1007/s00259-019-04400-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-019-04400-w