Abstract
Purpose
Grade 3 NENs are aggressive tumours with poor prognosis. PRRT+/− radiosensitising chemotherapy is a potential treatment for disease with high somatostatin receptor (SSTR) expression without spatially discordant FDG-avid disease. We retrospectively evaluated the efficacy of PRRT in G3 NEN.
Methods
Kaplan–Meier estimation was used to determine progression-free survival (PFS) and overall survival (OS) defined from start of PRRT. Subgroup analysis was performed for patients with Ki-67 ≤ 55% and >55%. Anatomical response (RECIST 1.1) and toxicity 3 months after PRRT was determined. Disease control rate (DCR) was defined as complete response (CR), partial response (PR) and stable disease (SD) of those with prior progression.
Results
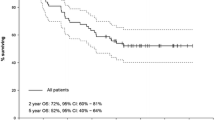
28 patients (M = 17; age 16–78 years; Ki-67 ≤ 55% = 22) were reviewed. 17 patients had pancreatic, 5 small bowel, 3 large bowel, 2 bronchial and 1 unknown primary disease. 25/28 had significant FDG-avid disease prior to treatment. Most had 177Lu-DOTA-octreotate (median cumulative activity 24.4 GBq, median 4 cycles). Twenty patients had radiosensitising chemotherapy. 89% were treated for disease progression; 79% after prior chemotherapy. Median follow-up was 29 months. The median PFS was 9 months for all patients. 16 patients died (Ki-67 ≤ 55% = 11; Ki-67 > 55% = 5) with median OS of 19 months. For Ki-67 ≤ 55% (N = 22), the median PFS was 12 months and median OS 46 months. For Ki-67 > 55% (N = 6), the median PFS was 4 months and median OS 7 months. On CT imaging, DCR at 3 months post-PRRT was 74%, 35% (8/23) PR and 39% (9/23) SD. Eleven patients received further PRRT due to recrudescent disease after response. Five patients developed progression of discordant FDG-avid disease and were referred for targeted therapy/chemotherapy. Grade 3 and 4 lymphopenia and thrombocytopenia occurred in five and five patients, respectively. No renal or liver toxicity related to treatment was seen.
Conclusions
PRRT achieves clinically relevant disease control with acceptable toxicity in G3 NENs.







Similar content being viewed by others
Change history
20 November 2017
On page 4 of the original version of this article, the text “Eight (29%) of the patients had significant FDG-avid disease (i.e. with intensity above liver parenchyma) prior to treatment” needs to be corrected.
References
Rindi G, Arnold R, Bosman FT, Bosman T, Carneiro F, Hruban R, et al. Nomenclature and classification of neuroendocrine neoplasms of the digestive system. WHO Classification of Tumours of the Digestive System 4th edn. Lyon: International Agency for Research on Cancer (IARC); 2010. 13–4.
Öberg K, Knigge U, Kwekkeboom D, Perren A, ESMO Guidelines Working Group. Neuroendocrine gastro-entero-pancreatic tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23:vii124–30.
Sorbye H, Welin S, Langer SW, Vestermark LW, Holt N, Osterlund P, et al. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): the NORDIC NEC study. Ann Oncol. 2013;24:152–60. https://doi.org/10.1093/annonc/mds276.
Fazio N, Spada F, Giovannini M. Chemotherapy in gastroenteropancreatic (GEP) neuroendocrine carcinomas (NEC): a critical view. Cancer Treat Rev. 2013;39:270–4. https://doi.org/10.1016/j.ctrv.2012.06.009.
Velayoudom-Cephise FL, Duvillard P, Foucan L, Hadoux J, Chougnet CN, Leboulleux S, et al. Are G3 ENETS neuroendocrine neoplasms heterogeneous? Endocr Relat Cancer. 2013;20:649–57. https://doi.org/10.1530/ERC-13-0027.
Heetfeld M, Chougnet CN, Olsen IH, Rinke A, Borbath I, Crespo G, et al. Characteristics and treatment of patients with G3 gastroenteropancreatic neuroendocrine neoplasms. Endocr Relat Cancer. 2015;22:657–64.
Milione M, Maisonneuve P, Spada F, Pellegrinelli A, Spaggiari P, Albarello L, et al. The clinicopathologic heterogeneity of grade 3 gastroenteropancreatic neuroendocrine neoplasms: morphological differentiation and proliferation identify different prognostic categories. Neuroendocrinology. 2017;104(1):85–93.
Tang LH, Basturk O, Sue JJ, Klimstra DS. A practical approach to the classification of WHO grade 3 (G3) well-differentiated Neuroendocrine tumor (WD-NET) and poorly differentiated Neuroendocrine carcinoma (PD-NEC) of the pancreas. Am J Surg Pathol. 2016;40(9):1192–202. https://doi.org/10.1097/PAS.0000000000000662.
Tang LH, Untch BR, Reidy DL, O'Reilly E, Dhall D, Jih L, et al. Well-differentiated Neuroendocrine tumors with a morphologically apparent high-grade component: a pathway distinct from poorly differentiated Neuroendocrine carcinomas. Clin Cancer Res. 2016;22(4):1011–7. https://doi.org/10.1158/1078-0432.CCR-15-0548.
Hofman MS, Hicks RJ. Changing paradigms with molecular imaging of neuroendocrine tumors. Discov Med. 2012;14(74):71–81.
Binderup T, Knigge U, Loft A, Federspiel B, Kjaer A. 18F-Fluorodeoxyglucose positron emission tomography predicts survival of patients with neuroendocrine tumors. Clin Cancer Res. 2010;16(3):978–85. https://doi.org/10.1158/1078-0432.CCR-09-1759.
Garin E, Le Jeune F, Devillers A, Cuggia M, de Lajarte-Thirouard AS, Bouriel C, et al. Predictive value of 18F-FDG PET and somatostatin receptor scintigraphy in patients with metastatic endocrine tumors. J Nucl Med. 2009;50(6):858–64. https://doi.org/10.2967/jnumed.108.057505.
Hicks RJ, Kwekkeboom DJ, Krenning E, Bodei L, Grozinsky-Glasberg S, Arnold R, et al. ENETS consensus guidelines for the standards of Care in Neuroendocrine Neoplasia: peptide receptor radionuclide therapy with Radiolabeled Somatostatin analogues. Neuroendocrinology. 2017; https://doi.org/10.1159/000475526.
Kashyap R, Hofman MS, Michael M, Kong G, Akhurst T, Eu P, et al. Favourable outcomes of (177)Lu-octreotate peptide receptor chemoradionuclide therapy in patients with FDG-avid neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2015;42(2):176–85. https://doi.org/10.1007/s00259-014-2906-4.
Armaghany T, Vahdati G, Thamake S, Hamidi M, Amerinia R, Delpassand E. Treatment of high grade metastatic neuroendocrine tumor (mNET) with peptide receptor radionuclide therapy (PRRT): Retrospective analysis in a single referral center. J Clin Oncol 33, 2015 (suppl; abstr e15175).
Ezziddin S, Opitz M, Attassi M, Biermann K, Sabet A, Guhlke S, et al. Impact of the Ki-67 proliferation index on response to peptide receptor radionuclide therapy. Eur J Nucl Med Mol Imaging. 2011;38(3):459–66. https://doi.org/10.1007/s00259-010-1610-2.
Garcia-Carbonero R, Sorbye H, Baudin E, Raymond E, Wiedenmann B, Niederle B, et al. ENETS consensus guidelines for high-grade gastroenteropancreatic neuroendocrine tumors and neuroendocrine carcinomas. Neuroendocrinology. 2016;103:186–94. https://doi.org/10.1159/000443172.
Kwekkeboom DJ, de Herder WW, Kam BL, van Eijck CH, van Essen M, Kooij PP, et al. Treatment with the radiolabeled somatostatin analog [177Lu-DOTA0,Tyr3]octreotate: toxicity, efficacy, and survival. J Clin Oncol. 2008;26:2124–30.
Hofman MS, Hicks RJ. Peptide receptor radionuclide therapy for neuroendocrine tumours: standardized and randomized, or personalized? Eur J Nucl Med Mol Imaging. 2014;41(2):211–3. https://doi.org/10.1007/s00259-013-2621-6.
Kong G, Callahan J, Hofman MS, Pattison DA, Akhurst T, Michael M, et al. High clinical and morphologic response using 90Y-DOTA-octreotate sequenced with 177Lu-DOTA-octreotate induction peptide receptor chemoradionuclide therapy (PRCRT) for bulky neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2017;44(3):476–89. https://doi.org/10.1007/s00259-016-3527-x.
Hofman MS, Michael M, Hicks RJ. 177Lu-Dotatate for Midgut Neuroendocrine tumors. N Engl J Med. 2017;376(14):1390–1. https://doi.org/10.1056/NEJMc1701616.
Bodei L, Mueller-Brand J, Baum RP, Pavel ME, Hörsch D, O'Dorisio MS, et al. The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2013;40(5):800–16. https://doi.org/10.1007/s00259-012-2330-6.
Kashyap R, Jackson P, Hofman MS, Eu P, Beauregard JM, Zannino D, et al. Rapid blood clearance and lack of long-term renal toxicity of 177Lu-DOTATATE enables shortening of renoprotective amino acid infusion. Eur J Nucl Med Mol Imaging. 2013;40(12):1853–60. https://doi.org/10.1007/s00259-013-2504-x.
Kong G, Thompson M, Collins M, Herschtal A, Hofman MS, Johnston V, et al. Assessment of predictors of response and longterm survival of patients with neuroendocrine tumour treated with peptide receptor chemoradionuclide therapy (PRCRT). Eur J Nucl Med Mol Imaging. 2014;41(10):1831–44. https://doi.org/10.1007/s00259-014-2788-5.
Kong G, Johnston V, Ramdave S, Lau E, Rischin D, Hicks RJ. High-administered activity in-111 octreotide therapy with concomitant radiosensitizing 5FU chemotherapy for treatment of neuroendocrine tumors: preliminary experience. Cancer Biother Radiopharm. 2009;24(5):527–33. https://doi.org/10.1089/cbr.2009.0644.
Hubble D, Kong G, Michael M, Johnson V, Ramdave S, Hicks RJ. 177Lu-Octreotate, alone or with radiosensitising chemotherapy, is safe in neuroendocrine tumour patients previously treated with high-activity 111In-octreotide. Eur J Nucl Med Mol Imaging. 2010;37(10):1869–75. https://doi.org/10.1007/s00259-010-1483-4.
Claringbold PG, Brayshaw PA, Price RA, Turner JH. Phase II study of radiopeptide 177Lu-octreotate and capecitabine therapy of progressive disseminated neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2011;38(2):302–11. https://doi.org/10.1007/s00259-010-1631-x.
Fine RL, Gulati AP, Krantz BA, Moss RA, Schreibman S, Tsushima DA, et al. Capecitabine and temozolomide (CAPTEM) for metastatic, well-differentiated neuroendocrine cancers: the pancreas Center at Columbia University experience. Cancer Chemother Pharmacol. 2013;71(3):663–70. https://doi.org/10.1007/s00280-012-2055-z.
Strosberg JR, Fine RL, Choi J, Nasir A, Coppola D, Chen DT, et al. First-line chemotherapy with capecitabine and temozolomide in patients with metastatic pancreatic endocrine carcinomas. Cancer. 2011;117(2):268–75. https://doi.org/10.1002/cncr.25425.
Claringbold PG, Price RA, Turner JH. Phase I-II study of radiopeptide 177Lu-octreotate in combination with capecitabine and temozolomide in advanced low-grade neuroendocrine tumors. Cancer Biother Radiopharm. 2012;27(9):561–9. https://doi.org/10.1089/cbr.2012.1276.
Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50(Suppl 1):122S–50S. https://doi.org/10.2967/jnumed.108.057307.
Hicks RJ. The role of PET in monitoring therapy. Cancer Imaging. 2005;5:51–7.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47. https://doi.org/10.1016/j.ejca.2008.10.026.
Rindi G. The ENETS guidelines: the new TNM classification system. Tumori. 2010;96(5):806–9.
Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, et al. Phase 3 trial of 177Lu-Dotatate for Midgut Neuroendocrine tumors. N Engl J Med. 2017;376(2):125–35. https://doi.org/10.1056/NEJMoa1607427.
Strosberg JR, Coppola D, Klimstra DS, Phan AT, Kulke MH, Wiseman GA, et al. The NANETS consensus guidelines for the diagnosis and management of poorly differentiated (high-grade) extrapulmonary neuroendocrine carcinomas. Pancreas. 2010;39:799–800. https://doi.org/10.1097/MPA.0b013e3181ebb56f.
Welin S, Sorbye H, Sebjornsen S, Knappskog S, Busch C, Oberg K. Clinical effect of temozolomide-based chemotherapy in poorly differentiated endocrine carcinoma after progression on first-line chemotherapy. Cancer 2011; 11: 4617–4622. doi: https://doi.org/10.1002/cncr.26124.
Panzuto F, Rinzivillo M, Spada F, Antonuzzo L, Ibrahim T, Campana D, et al. Everolimus in pancreatic Neuroendocrine carcinomas G3. Pancreas. 2017;46(3):302–5. https://doi.org/10.1097/MPA.0000000000000762.
Kesavan M, Claringbold PG, Turner JH. Hematological toxicity of combined 177Lu-octreotate radiopeptide chemotherapy of gastroenteropancreatic neuroendocrine tumors in long-term follow-up. Neuroendocrinology. 2014;99(2):108–17. https://doi.org/10.1159/000362558.
Acknowledgements
Professor Hicks’ research is supported by a National Health and Medical Research Council of Australia Program grant and practitioner fellowship. We thank our radiopharmacists and radiochemists for their excellent support of our theranostics program and our dedicated nuclear medicine technologists and nursing staff for the care of our patients. Finally, we are grateful for the trust invested in us by our patients, their families and their managing clinicians.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflicts of interest. No funding was received. All procedures performed were in accordance with the ethical standards of the institutional research committee and all patients provided informed consent for treatment.
Additional information
Sue Ping Thang and Mei Sim Lung are co-first authors.
A correction to this article is available online at https://doi.org/10.1007/s00259-017-3886-y.
Rights and permissions
About this article
Cite this article
Thang, S.P., Lung, M.S., Kong, G. et al. Peptide receptor radionuclide therapy (PRRT) in European Neuroendocrine Tumour Society (ENETS) grade 3 (G3) neuroendocrine neoplasia (NEN) - a single-institution retrospective analysis. Eur J Nucl Med Mol Imaging 45, 262–277 (2018). https://doi.org/10.1007/s00259-017-3821-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-017-3821-2