Abstract
Purpose
The updated Winter nomogram is the only nomogram predicting lymph node invasion (LNI) in prostate cancer (PCa) patients based on sentinel node (SN) dissection (sLND). The aim of the study was to externally validate the Winter nomogram and examine its performance in patients undergoing extended pelvic lymph node dissection (ePLND), ePLND combined with SN biopsy (SNB) and sLND only. The results were compared with the Memorial Sloan Kettering Cancer Center (MSKCC) and updated Briganti nomograms.
Methods
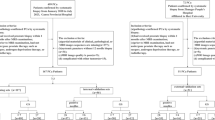
This retrospective study included 1183 patients with localized PCa undergoing robot-assisted laparoscopic radical prostatectomy (RARP) combined with pelvic lymphadenectomy and 224 patients treated with sLND and external beam radiotherapy (EBRT), aiming to offer pelvic radiotherapy only in case of histologically positive SNs. In the RARP population, ePLND was applied in 956 (80.8%) patients,while 227 (19.2%) patients were offered ePLND combined with additional SNB.
Results
The median numbers of removed nodes were 10 (interquartile range, IQR = 6-14), 15 (IQR = 10-20) and 7 (IQR = 4-10) in the ePLND, ePLND + SNB, and sLND groups, respectively. Corresponding LNI rates were 16.6%, 25.5% and 42%. Based on the AUC, the performance of the Briganti nomogram (0.756) in the ePLND group was superior to both the MSKCC (0.744) and Winter nomogram (0.746). The Winter nomogram, however, was the best predictor of LNI in both the ePLND + SNB (0.735) and sLND (0.709) populations. In the calibration analysis, all nomograms showed better accuracy in the low/intermediate risk patients, while in the high-risk population, an overestimation of the risk for LNI was observed.
Conclusion
The SN-based updated nomogram showed better prediction in the SN population. The results were also comparable, relative to predictive tools developed with (e)PLND, suggesting a difference in sampling accuracy between SNB and non-SNB. Patients who benefit most from the nomogram would be those with a low/intermediate risk of LN metastasis.







Similar content being viewed by others
References
Budäus L, Leyh-Bannurah SR, Salomon G, Michl U, Heinzer H, Huland H, et al. Initial experience of (68)Ga-PSMA PET/CT imaging in high-risk prostate cancer patients prior to radical prostatectomy. Eur Urol. 2016;69(3):393–6.
Briganti A, Chun FK, Salonia A, Zanni G, Scattoni V, Valiquette L, et al. Validation of a nomogram predicting the probability of lymph node invasion among patients undergoing radical prostatectomy and an extended pelvic lymphadenectomy. Eur Urol. 2006;49(6):1019–26. discussion 1026-7
Touijer K, Rabbani F, Otero JR, Secin FP, Eastham JA, Scardino PT, et al. Standard versus limited pelvic lymph node dissection for prostate cancer in patients with a predicted probability of nodal metastasis greater than 1%. J Urol. 2007;178(1):120–4.
Mottet N, Bellmunt J, Bolla M, Briers E, Cumberbatch MG, De Santis M, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2017;71(4):618–29.
Fossati N, Willemse PM, Van den Broeck T, van den Bergh RC, Yuan CY, Briers E, et al. The benefits and harms of different extents of lymph node dissection during radical prostatectomy for prostate cancer: a systematic review. Eur Urol. 2017;72(1):84–109.
Joniau S, Van den Bergh L, Lerut E, Deroose CM, Haustermans K, Oyen R, et al. Mapping of pelvic lymph node metastases in prostate cancer. Eur Urol. 2013;63(3):450–8.
Briganti A, Blute ML, Eastham JH, Graefen M, Heidenreich A, Karnes JR, et al. Pelvic lymph node dissection in prostate cancer. Eur Urol. 2009;55(6):1251–65.
Briganti A, Karakiewicz PI, Chun FK, Gallina A, Salonia A, Zanni G, et al. Percentage of positive biopsy cores can improve the ability to predict lymph node invasion in patients undergoing radical prostatectomy and extended pelvic lymph node dissection. Eur Urol. 2007;51(6):1573–81.
Godoy G, Chong KT, Cronin A, Vickers A, Laudone V, Touijer K, et al. Extent of pelvic lymph node dissection and the impact of standard template dissection on nomogram prediction of lymph node involvement. Eur Urol. 2011;60(2):195–201.
Heidenreich A, Pfister D, Thüer D, Brehmer B. Percentage of positive biopsies predicts lymph node involvement in men with low-risk prostate cancer undergoing radical prostatectomy and extended pelvic lymphadenectomy. BJU Int. 2011;107(2):220–5.
Briganti A, Larcher A, Abdollah F, Capitanio U, Gallina A, Suardi N, et al. Updated nomogram predicting lymph node invasion in patients with prostate cancer undergoing extended pelvic lymph node dissection: the essential importance of percentage of positive cores. Eur Urol. 2012;61(3):480–7.
Shariat SF, Karakiewicz PI, Suardi N, Kattan MW. Comparison of nomograms with other methods for predicting outcomes in prostate cancer: a critical analysis of the literature. Clin Cancer Res. 2008;14(14):4400–7.
Schmitges J, Karakiewicz PI, Sun M, Abdollah F, Budäus L, Isbarn H, et al. Predicting the risk of lymph node invasion during radical prostatectomy using the European Association of Urology guideline nomogram: a validation study. Eur J Surg Oncol. 2012;38(7):624–9.
Hansen J, Rink M, Bianchi M, Kluth LA, Tian Z, Ahyai SA, et al. External validation of the updated Briganti nomogram to predict lymph node invasion in prostate cancer patients undergoing extended lymph node dissection. Prostate. 2013;73(2):211–8.
Gacci M, Schiavina R, Lanciotti M, Masieri L, Serni S, Vagnoni V, et al. External validation of the updated nomogram predicting lymph node invasion in patients with prostate cancer undergoing extended pelvic lymph node dissection. Urol Int. 2013;90(3):277–82.
Winter A, Kneib T, Henke RP, Wawroschek F. Sentinel lymph node dissection in more than 1200 prostate cancer cases: rate and prediction of lymph node involvement depending on preoperative tumor characteristics. Int J Urol. 2014;21(1):58–63.
Dell'Oglio P, Abdollah F, Suardi N, Gallina A, Cucchiara V, Vizziello D, et al. External validation of the European association of urology recommendations for pelvic lymph node dissection in patients treated with robot-assisted radical prostatectomy. J Endourol. 2014;28(4):416–23.
Schubert T, Uphoff J, Henke RP, Wawroschek F, Winter A. Reliability of radioisotope-guided sentinel lymph node biopsy in penile cancer: verification in consideration of the European guidelines. BMC Urol. 2015;15:98.
van der Poel HG, Meershoek P, Grivas N, KleinJan G, van Leeuwen FW, Horenblas S. Sentinel node biopsy and lymphatic mapping in penile and prostate cancer. Urologe A. 2017;56(1):13–7.
Wit EM, Acar C, Grivas N, Yuan C, Horenblas S, Liedberg F, et al. Sentinel node procedure in prostate cancer: a systematic review to assess diagnostic accuracy. Eur Urol. 2017;71(4):596–605.
KleinJan GH, van den Berg NS, Brouwer OR, de Jong J, Acar C, Wit EM, et al. Optimisation of fluorescence guidance during robot-assisted laparoscopic sentinel node biopsy for prostate cancer. Eur Urol. 2014;66(6):991–8.
Acar C, Kleinjan GH, van den Berg NS, Wit EM, van Leeuwen FW, van der Poel HG. Advances in sentinel node dissection in prostate cancer from a technical perspective. Int J Urol. 2015;22(10):898–909.
Winter A, Kneib T, Rohde M, Henke RP, Wawroschek F. First nomogram predicting the probability of lymph node involvement in prostate cancer patients undergoing radioisotope guided sentinel lymph node dissection. Urol Int. 2015;95(4):422–8.
Winter A, Kneib Th, Wasylow C, Reinhardt L, Henke RP, Engels S, et al. Updated nomogram incorporating percentage of positive cores to predict probability of lymph node invasion in prostate cancer patients undergoing sentinel lymph node dissection. J Cancer. 2017. doi:10.7150/jca.20409.
Memorial Sloan Kettering Cancer Center. Prostate Cancer Nomograms Pre-Radical Prostatectomy. (2016) Available at: https://www.mskcc.org/nomograms/prostate/pre-op/coefficients. Accessed December 20, 2016.
Grivas N, Wit E, Pos F, de Jong J, Vegt E, Bex A, et al. Sentinel lymph node dissection to select clinically node-negative prostate cancer patients for pelvic radiation therapy: effect on biochemical recurrence and systemic progression. Int J Radiat Oncol Biol Phys. 2017;97(2):347–54.
Kattan MW. Factors affecting the accuracy of prediction models limit the comparison of rival prediction models when applied to separate data sets. Eur Urol. 2011;59(4):566–7.
Mohler J, Bahnson RR, Boston B, Busby JE, D'Amico A, Eastham JA, et al. NCCN clinical practice guidelines in oncology: prostate cancer. J Natl Compr Cancer Netw. 2010;8(2):162–200.
Abdollah F, Klett DE, Sammon JD, Dalela D, Sood A, Hsu L, et al. Predicting lymph node invasion in patients treated with robot-assisted radical prostatectomy. Can J Urol. 2016;23(1):8141–50.
Briganti A, Capitanio U, Chun FK, Gallina A, Suardi N, Salonia A, et al. Impact of surgical volume on the rate of lymph node metastases in patients undergoing radical prostatectomy and extended pelvic lymph node dissection for clinically localized prostate cancer. Eur Urol. 2008;54(4):794–802.
Briganti A, Bianchi M, Sun M, Suardi N, Gallina A, Abdollah F, et al. Impact of the introduction of a robotic training programme on prostate cancer stage migration at a single tertiary referral centre. BJU Int. 2013;111(8):1222–30.
Holl G, Dorn R, Wengenmair H, Weckermann D, Sciuk J. Validation of sentinel lymph node dissection in prostate cancer: experience in more than 2,000 patients. Eur J Nucl Med Mol Imaging. 2009;36(9):1377–82.
Weckermann D, Dorn R, Holl G, Wagner T, Harzmann R. Limitations of radioguided surgery in high-risk prostate cancer. Eur Urol. 2007;51(6):1549–56.
Bertolini G, D'Amico R, Nardi D, Tinazzi A, Apolone G. One model, several results: the paradox of the Hosmer-Lemeshow goodness-of-fit test for the logistic regression model. J Epidemiol Biostat. 2000;5(4):251–3.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
None.
Conflicts of interest
All authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in this study were in accordance with the ethical standards of the institutional and national research committee and with the principles of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. All patients published in this manuscript provided written informed consent.
Electronic supplementary material
Supplementary Table 1
(DOCX 14 kb)
Supplementary Table 2
(DOCX 15 kb)
Supplementary Table 3
(DOCX 14 kb)
Supplementary Table 4
(DOCX 15 kb)
Rights and permissions
About this article
Cite this article
Grivas, N., Wit, E., Tillier, C. et al. Validation and head-to-head comparison of three nomograms predicting probability of lymph node invasion of prostate cancer in patients undergoing extended and/or sentinel lymph node dissection. Eur J Nucl Med Mol Imaging 44, 2213–2226 (2017). https://doi.org/10.1007/s00259-017-3788-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-017-3788-z