Abstract
Purpose
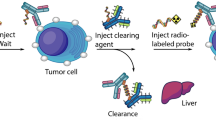
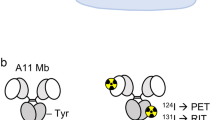
GPA33 is a colorectal cancer (CRC) antigen with unique retention properties after huA33-mediated tumor targeting. We tested a pretargeted radioimmunotherapy (PRIT) approach for CRC using a tetravalent bispecific antibody with dual specificity for GPA33 tumor antigen and DOTA-Bn–(radiolanthanide metal) complex.
Methods
PRIT was optimized in vivo by titrating sequential intravenous doses of huA33-C825, the dextran-based clearing agent, and the C825 haptens 177Lu-or 86Y-DOTA-Bn in mice bearing the SW1222 subcutaneous (s.c.) CRC xenograft model.
Results
Using optimized PRIT, therapeutic indices (TIs) for tumor radiation-absorbed dose of 73 (tumor/blood) and 12 (tumor/kidney) were achieved. Estimated absorbed doses (cGy/MBq) to tumor, blood, liver, spleen, and kidney for single-cycle PRIT were 65.8, 0.9 (TI 73), 6.3 (TI 10), 6.6 (TI 10), and 5.3 (TI 12), respectively. Two cycles of PRIT (66.6 or 111 MBq 177Lu-DOTA-Bn) were safe and effective, with a complete response of established s.c. tumors (100 – 700 mm3) in nine of nine mice, with two mice alive without recurrence at >140 days. Tumor log kill in this model was estimated to be 2.1 – 3.0 based on time to 500-mm3 tumor recurrence. In addition, PRIT dosimetry/diagnosis was performed by PET imaging of the positron-emitting DOTA hapten 86Y-DOTA-Bn.
Conclusion
We have developed anti-GPA33 PRIT as a triple-step theranostic strategy for preclinical detection, dosimetry, and safe targeted radiotherapy of established human colorectal mouse xenografts.




Similar content being viewed by others
References
American Cancer Society. Colorectal cancer facts & figures 2014–2016. Atlanta: American Cancer Society; 2014.
Larson SM, Carrasquillo JA, Cheung NK, Press OW. Radioimmunotherapy of human tumours. Nat Rev Cancer. 2015;15:347–60. doi:10.1038/nrc3925.
Hajjar G, Sharkey RM, Burton J, Zhang CH, Yeldell D, Matthies A, et al. Phase I radioimmunotherapy trial with iodine-131-labeled humanized MN-14 anti-carcinoembryonic antigen monoclonal antibody in patients with metastatic gastrointestinal and colorectal cancer. Clin Colorectal Cancer. 2002;2:31–42. doi:10.3816/CCC.2002.n.009.
Wong JYC, Chu DZ, Yamauchi DM, Williams LE, Liu A, Wilczynski S, et al. A phase I radioimmunotherapy trial evaluating 90yttrium-labeled anti-carcinoembryonic antigen (CEA) chimeric T84.66 in patients with metastatic CEA-producing malignancies. Clin Cancer Res. 2000;6:3855–63.
Goldenberg DM, Chang CH, Rossi EA, McBride JW, Sharkey RM. Pretargeted molecular imaging and radioimmunotherapy. Theranostics. 2012;2:523–40. doi:10.7150/thno.3582.
Hagan PL, Halpern SE, Dillman RO, Shawler DL, Johnson DE, Chen A, et al. Tumor size: effect on monoclonal antibody uptake in tumor models. J Nucl Med. 1986;27:422–7.
Mayer A, Tsiompanou E, Flynn AA, Pedley RB, Dearling J, Boden R, et al. Higher dose and dose-rate in smaller tumors result in improved tumor control. Cancer Invest. 2003;21:382–8.
O’Donoghue JA, Smith-Jones PM, Humm JL, Ruan S, Pryma DA, Jungbluth AA, et al. 124I-huA33 antibody uptake is driven by A33 antigen concentration in tissues from colorectal cancer patients imaged by immuno-PET. J Nucl Med. 2011;52:1878–85. doi:10.2967/jnumed.111.095596.
Stillebroer AB, Zegers CM, Boerman OC, Oosterwijk E, Mulders PF, O’Donoghue JA, et al. Dosimetric analysis of 177Lu-cG250 radioimmunotherapy in renal cell carcinoma patients: correlation with myelotoxicity and pretherapeutic absorbed dose predictions based on 111In-cG250 imaging. J Nucl Med. 2012;53:82–9. doi:10.2967/jnumed.111.094896.
O’Donoghue JA, Bardies M, Wheldon TE. Relationships between tumor size and curability for uniformly targeted therapy with beta-emitting radionuclides. J Nucl Med. 1995;36:1902–9.
Reardan DT, Meares CF, Goodwin DA, McTigue M, David GS, Stone MR, et al. Antibodies against metal chelates. Nature. 1985;316:265–8.
Goodwin DA, Meares CF, Watanabe N, McTigue M, Chaovapong W, Ransone CM, et al. Pharmacokinetics of pretargeted monoclonal antibody 2D12.5 and 88Y-Janus-2-(p-nitrobenzyl)-1,4,7,10-tetraazacyclododecanetetraacetic acid (DOTA) in BALB/c mice with KHJJ mouse adenocarcinoma: a model for 90Y radioimmunotherapy. Cancer Res. 1994;54:5937–46.
Feng X, Pak RH, Kroger LA, Moran JK, DeNardo DG, Meares CF, et al. New anti-Cu-TETA and anti-Y-DOTA monoclonal antibodies for potential use in the pre-targeted delivery of radiopharmaceuticals to tumor. Hybridoma. 1998;17:125–32.
Corneillie TM, Whetstone PA, Meares CF. Irreversibly binding anti-metal chelate antibodies: artificial receptors for pretargeting. J Inorg Biochem. 2006;100:882–90. doi:10.1016/j.jinorgbio.2006.01.004.
Chmura AJ, Orton MS, Meares CF. Antibodies with infinite affinity. Proc Natl Acad Sci U S A. 2001;98:8480–4. doi:10.1073/pnas.151260298.
Orcutt KD, Slusarczyk AL, Cieslewicz M, Ruiz-Yi B, Bhushan KR, Frangioni JV, et al. Engineering an antibody with picomolar affinity to DOTA chelates of multiple radionuclides for pretargeted radioimmunotherapy and imaging. Nucl Med Biol. 2011;38:223–33. doi:10.1016/j.nucmedbio.2010.08.013.
Orcutt KD, Ackerman ME, Cieslewicz M, Quiroz E, Slusarczyk AL, Frangioni JV, et al. A modular IgG-scFv bispecific antibody topology. Protein Eng Des Sel. 2010;23:221–8. doi:10.1093/protein/gzp077.
Cheal SM, Xu H, Guo HF, Zanzonico PB, Larson SM, Cheung NK. Preclinical evaluation of multistep targeting of diasialoganglioside GD2 using an IgG-scFv bispecific antibody with high affinity for GD2 and DOTA metal complex. Mol Cancer Ther. 2014;13:1803–12. doi:10.1158/1535-7163.MCT-13-0933.
Garinchesa P, Sakamoto J, Welt S, Real F, Rettig W, Old L. Organ-specific expression of the colon cancer antigen A33, a cell surface target for antibody-based therapy. Int J Oncol. 1996;9:465–71.
Welt S, Divgi CR, Kemeny N, Finn RD, Scott AM, Graham M, et al. Phase I/II study of iodine 131-labeled monoclonal antibody A33 in patients with advanced colon cancer. J Clin Oncol. 1994;12:1561–71.
King DJ, Antoniw P, Owens RJ, Adair JR, Haines AM, Farnsworth AP, et al. Preparation and preclinical evaluation of humanised A33 immunoconjugates for radioimmunotherapy. Br J Cancer. 1995;72:1364–72.
Carrasquillo JA, Pandit-Taskar N, O’Donoghue JA, Humm JL, Zanzonico P, Smith-Jones PM, et al. (124)I-huA33 antibody PET of colorectal cancer. J Nucl Med. 2011;52:1173–80. doi:10.2967/jnumed.110.086165.
Zanzonico P, Carrasquillo JA, Pandit-Taskar N, O’Donoghue JA, Humm JL, Smith-Jones P, et al. PET-based compartmental modeling of (124)I-A33 antibody: quantitative characterization of patient-specific tumor targeting in colorectal cancer. Eur J Nucl Med Mol Imaging. 2015;42:1700–6. doi:10.1007/s00259-015-3061-2.
Erdi YE, Macapinlac H, Larson SM, Erdi AK, Yeung H, Furhang EE, et al. Radiation dose assessment for I-131 therapy of thyroid cancer using I-124 PET imaging. Clin Positron Imaging. 1999;2:41–6.
Kolbert KS, Pentlow KS, Pearson JR, Sheikh A, Finn RD, Humm JL, et al. Prediction of absorbed dose to normal organs in thyroid cancer patients treated with 131I by use of 124I PET and 3-dimensional internal dosimetry software. J Nucl Med. 2007;48:143–9.
Ho AL, Grewal RK, Leboeuf R, Sherman EJ, Pfister DG, Deandreis D, et al. Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer. N Engl J Med. 2013;368:623–32. doi:10.1056/NEJMoa1209288.
Barendswaard EC, Humm JL, O’Donoghue JA, Sgouros G, Finn RD, Scott AM, et al. Relative therapeutic efficacy of (125)I- and (131)I-labeled monoclonal antibody A33 in a human colon cancer xenograft. J Nucl Med. 2001;42:1251–6.
Ruan S, O’Donoghue JA, Larson SM, Finn RD, Jungbluth A, Welt S, et al. Optimizing the sequence of combination therapy with radiolabeled antibodies and fractionated external beam. J Nucl Med. 2000;41:1905–12.
Barendswaard EC, O’Donoghue JA, Larson SM, Tschmelitsch J, Welt S, Finn RD, et al. 131I radioimmunotherapy and fractionated external beam radiotherapy: comparative effectiveness in a human tumor xenograft. J Nucl Med. 1999;40:1764–8.
Antoniw P, Farnsworth AP, Turner A, Haines AM, Mountain A, Mackintosh J, et al. Radioimmunotherapy of colorectal carcinoma xenografts in nude mice with yttrium-90 A33 IgG and Tri-Fab (TFM). Br J Cancer. 1996;74:513–24.
El Emir E, Qureshi U, Dearling JL, Boxer GM, Clatworthy I, Folarin AA, et al. Predicting response to radioimmunotherapy from the tumor microenvironment of colorectal carcinomas. Cancer Res. 2007;67:11896–905. doi:10.1158/0008-5472.CAN-07-2967.
Orcutt KD, Rhoden JJ, Ruiz-Yi B, Frangioni JV, Wittrup KD. Effect of small-molecule-binding affinity on tumor uptake in vivo: a systematic study using a pretargeted bispecific antibody. Mol Cancer Ther. 2012;11:1365–72. doi:10.1158/1535-7163.MCT-11-0764.
McDevitt MR, Chattopadhyay D, Jaggi JS, Finn RD, Zanzonico PB, Villa C, et al. PET imaging of soluble yttrium-86-labeled carbon nanotubes in mice. PLoS One. 2007;2:e907. doi:10.1371/journal.pone.0000907.
Orcutt KD, Nasr KA, Whitehead DG, Frangioni JV, Wittrup KD. Biodistribution and clearance of small molecule hapten chelates for pretargeted radioimmunotherapy. Mol Imaging Biol. 2011;13:215–21. doi:10.1007/s11307-010-0353-6.
Marks LB, Yorke ED, Jackson A, Ten Haken RK, Constine LS, Eisbruch A, et al. Use of normal tissue complication probability models in the clinic. Int J Radiat Oncol Biol Phys. 2010;76:S10–9. doi:10.1016/j.ijrobp.2009.07.1754.
Beauregard JM, Hofman MS, Pereira JM, Eu P, Hicks RJ. Quantitative (177)Lu SPECT (QSPECT) imaging using a commercially available SPECT/CT system. Cancer Imaging. 2011;11:56–66. doi:10.1102/1470-7330.2011.0012.
Pentlow KS, Finn RD, Larson SM, Erdi YE, Beattie BJ, Humm JL. Quantitative imaging of yttrium-86 with PET: the occurrence and correction of anomalous apparent activity in high density regions. Clin Positron Imaging. 2000;3:85–90.
Beattie BJ, Finn RD, Rowland DJ, Pentlow KS. Quantitative imaging of bromine-76 and yttrium-86 with PET: a method for the removal of spurious activity introduced by cascade gamma rays. Med Phys. 2003;30:2410–23.
Schmidt MM, Wittrup KD. A modeling analysis of the effects of molecular size and binding affinity on tumor targeting. Mol Cancer Ther. 2009;8:2861–71. doi:10.1158/1535-7163.MCT-09-0195.
Wittrup KD, Thurber GM, Schmidt MM, Rhoden JJ. Practical theoretic guidance for the design of tumor-targeting agents. Methods Enzymol. 2012;503:255–68. doi:10.1016/B978-0-12-396962-0.00010-0.
Yazaki PJ, Lee B, Channappa D, Cheung CW, Crow D, Chea J, et al. A series of anti-CEA/anti-DOTA bispecific antibody formats evaluated for pre-targeting: comparison of tumor uptake and blood clearance. Protein Eng Des Sel. 2013;26:187–93. doi:10.1093/protein/gzs096.
Liu G, Hnatowich DJ. A semiempirical model of tumor pretargeting. Bioconjug Chem. 2008;19:2095–104. doi:10.1021/bc8002748.
Acknowledgments
The authors thank Donald Axworthy, Dr. Kelly Orcutt, and Dr. James Russell for helpful discussions. The authors gratefully acknowledge Teja Muralidhar Kalidindi, Valerie Longo, and the Memorial Sloan Kettering Small Animal Imaging Core Facility for support with experiments. The authors also thank Leah Bassity for her editorial work on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was supported in part by the following: Donna & Benjamin M. Rosen Chair (to S.M. Larson), Enid A. Haupt Chair (to N.K. Cheung), The Center for Targeted Radioimmunotherapy and Theranostics, Ludwig Center for Cancer Immunotherapy, Memorial Sloan Kettering Cancer Center (to S.M. Larson), a training grant from the National Institutes of Health (R25-CA096945; principal investigator H. Hricak, fellow S.M. Cheal), and a National Institutes of Health grant (R01-CA-101830; to K.D. Wittrup). S.M. Larson was also supported in part by P50-CA86438. Technical services provided by the Memorial Sloan Kettering Small-Animal Imaging Core Facility were supported by National Institutes of Health grants R24-CA83084 (to H. Hricak), P30-CA08748 (to C. Thompson), and P50-CA92629 (to H. Scher). National Institutes of Health Shared Instrumentation grant No. 1 S10 RR028889-01 (to P.B. Zanzonico), and a Shared Resources Grant from the Memorial Sloan Kettering Cancer Center Metastasis Research Center (to P. B. Zanzonico), which provided funding support for the purchase of the Focus 120 microPET and the NanoSPECT/CT Plus, respectively, are gratefully acknowledged.
Conflicts of interest
None.
Ethical approval
All animal experiments were approved by the Institutional Animal Care and Use Committee of Memorial Sloan Kettering Cancer Center, and institutional guidelines for the proper and humane use of animals in research were followed.
Additional information
Nai-Kong V. Cheung and Steven M. Larson are senior coauthors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 1336 kb)
Rights and permissions
About this article
Cite this article
Cheal, S.M., Xu, H., Guo, Hf. et al. Theranostic pretargeted radioimmunotherapy of colorectal cancer xenografts in mice using picomolar affinity 86Y- or 177Lu-DOTA-Bn binding scFv C825/GPA33 IgG bispecific immunoconjugates. Eur J Nucl Med Mol Imaging 43, 925–937 (2016). https://doi.org/10.1007/s00259-015-3254-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-015-3254-8