Abstract
Purpose
It has long been debated whether human cytomegalovirus (HCMV) and Epstein-Barr virus (EBV) are associated with rectal cancer. The gene products of HCMV and EBV contribute to cell-cycle progression, mutagenesis, angiogenesis and immune evasion. The aim of this prospective study was to analyse the association between infection of a tumour by HCMV and EBV and clinical, histological, metabolic (18F-FDG uptake), volumetric (from CT) and molecular (KRAS status) features and long-term outcomes in a homogeneously treated group of patients with locally advanced rectal cancer.
Methods
HCMV and EBV were detected in pretreatment biopsies using polymerase chain reaction (PCR). The Cox proportional hazards regression model was used to explore associations between viral infection and disease-free survival (DFS) and overall survival (OS).
Results
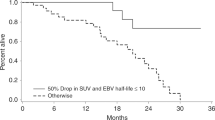
We analysed 37 patients with a median follow-up of 74 months (range 5–173 months). Locoregional control, OS and DFS at 5 years were 93 %, 74 % and 71 %, respectively. Patients with HCMV/EBV coinfection had a significantly higher maximum standardized uptake value than patients without viral coinfection (p = 0.02). Significant differences were also observed in staging and percentage relative reduction in tumour volume between patients with and without HCMV infection (p < 0.01) and EBV infection (p < 0.01). KRAS wildtype status was significantly more frequently observed in patients with EBV infection (p <0.01) and HCMV/EBV co-infection (p = 0.04). No significant differences were observed in OS or DFS between patients with and without EBV infection (p = 0.88 and 0.73), HCMV infection (p = 0.84 and 0.79), and EBV/CMV coinfection (p = 0.24 and 0.39).
Conclusion
This pilot study showed that viral infections were associated with metabolic staging differences, and differences in the evolution of metabolic and volumetric parameters and KRAS mutations. Further findings of specific features will help determine the best candidates for metabolic and volumetric staging and restaging. Further toxicity profile findings will help to determine the best candidates for specific supportive treatment during pelvic chemoradiotherapy in patients with locally advanced rectal cancer.

Similar content being viewed by others
References
Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet. 1986;1:1479–82.
Guckenberger M, Saur G, Wehner D, Sweeney RA, Thalheimer A, Germer CT, et al. Comparison of preoperative short course radiotherapy and long-course radiochemotherapy for locally advanced rectal cancer. Strahlenther Onkol. 2012;188:551–7.
Rödel C, Arnold D, Becker H, Fietkau R, Ghadimi M, Graeven U, et al. Induction chemotherapy before chemoradiotherapy and surgery for locally advanced rectal cancer: is it time for a randomized phase III trial. Strahlenther Onkol. 2010;186:658–64.
Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355:1114–23.
van Gijn W, Marijnen CA, Nagtegaal ID, Kranenbarg EM, Putter H, Wiggers T, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011;12:575–82.
Bujko K, Nowacki MP, Nasierowska-Guttmejer A, Michalski W, Bebenek M, Pudełko M, et al. Sphincter preservation following preoperative radiotherapy for rectal cancer: report of a randomised trial comparing short-term radiotherapy vs. conventionally fractionated radiochemotherapy. Radiother Oncol. 2004;72:15–24.
Blomqvist L, Glimelius B. The “good”, the “bad”, and the “ugly” rectal cancers. Acta Oncol. 2008;47:5–8.
Roth AD, Delorenzi M, Tejpar S, Yan P, Klingbiel D, Fiocca R, et al. Integrated analysis of molecular and clinical prognostic factors in stage II/III colon cancer. J Natl Cancer Inst. 2012;104:1635–46.
Calvo FA, Sole CV, de la Mata D, Cabezón L, Gómez-Espí M, Alvarez E, et al. 18F-FDG PET/CT-based treatment response evaluation in locally advanced rectal cancer: a prospective validation of long-term outcomes. Eur J Nucl Med Mol Imaging. 2013;40:657–67
Guillem JG, Ruby JA, Leibold T, Akhurst TJ, Yeung HW, Gollub MJ, et al. Neither FDG-PET nor CT can distinguish between a pathological complete response and an incomplete response after neoadjuvant chemoradiation in locally advanced rectal cancer: a prospective study. Ann Surg. 2013;258:289–95.
Antonic V, Stojadinovic A, Kester KE. Significance of infectious agents in colorectal cancer development. J Cancer Educ. 2013;4:227–40.
Michaelis M, Doerr HW, Cinatl J. The story of human cytomegalovirus and cancer: increasing evidence and open questions. Neoplasia. 2009;11:1–9.
Ma BB, King A, Lo YM, Yau YY, Zee B, Hui EP, et al. Relationship between pretreatment level of plasma Epstein-Barr virus DNA, tumor burden, and metabolic activity in advanced nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2006;66:714–20.
Chang KP, Tsang NM, Liao CT, Hsu CL, Chung MJ, Lo CW, et al. Prognostic significance of 18F-FDG PET parameters and plasma Epstein-Barr virus DNA load in patients with nasopharyngeal carcinoma. J Nucl Med. 2012;53:21–8.
Quirke P, Durdey P, Dixon MF, Williams NS. Local recurrence of rectal adenocarcinoma due to inadequate surgical resection. Histopathological study of lateral tumor spread and surgical excision. Lancet. 1986;2:996–9.
Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A AJCC cancer staging handbook, 7th ed. Heidelberg: Springer; 2007. p. 1–39.
Rödel C, Martus P, Papadoupolos T, Füzesi L, Klimpfinger M, Fietkau R, et al. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol. 2005;23:8688–96.
Beaulieu S, Kinahan P, Tseng J, Dunnwald LK, Schubert EK, Pham P, et al. SUV varies with time after injection in 18F-FDG PET of breast cancer: characterization and method to adjust for time differences. J Nucl Med. 2003;44:1044–50.
Boellaard R, Krak NC, Hoekstra OS, Lammertsma AA. Effects of noise, image resolution, and ROI definition on the accuracy of standard uptake values: a simulation study. J Nucl Med. 2004;45:1519–27.
Calvo FA, Cabezón L, González C, Soria A, de la Mata D, Gómez-Espí M, et al. PET imaging in rectal cancer 18F-FDG PET bio-metabolic monitoring of neoadjuvant therapy effects in rectal cancer: focus on nodal disease characteristics. Radiother Oncol. 2010;97:212–6.
Hong R, Lim SC. 18F-fluoro-2-deoxyglucose uptake on PET CT and glucose transporter 1 expression in colorectal adenocarcinoma. World J Gastroenterol. 2012;18:168–74.
Kawada K, Nakamoto Y, Kawada M, Hida K, Matsumoto T, Murakami T, et al. Relationship between 18Ffluorodeoxyglucose accumulation and KRAS/BRAF mutations in colorectal cancer. Clin Cancer Res. 2012;18:1696–703.
Tian M, Yu L, Zhang Y, Gao X. Correlations between SUVmax and expression of GLUT1 and growth factors inducing lymphangiogenesis. Acad Radiol. 2012;19:420–6.
Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tân PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35.
Blake MA, Singh A, Setty BN, Slattery J, Kalra M, Maher MM, et al. Pearls and pitfalls in interpretation of abdominal and pelvic PET-CT. Radiographics. 2006;26:1335–53.
Wanebo HJ, Antoniuk P, Koness RJ, Levy A, Vezeridis M, Cohen SI, et al. Pelvic resection of recurrent rectal cancer: technical considerations and outcomes. Dis Colon Rectum. 1999;42:1438–48.
Burnet NG, Barnett GC, Elliott RM, Dearnaley DP, Pharoah PD, Dunning AM, et al. RAPPER: the radiogenomics of radiation toxicity. Clin Oncol (R Coll Radiol). 2013;25:431–4.
Glynne-Jones R, Anyamene N. Just how useful an endpoint is complete pathological response after neoadjuvant chemoradiation in rectal cancer? Int J Radiat Oncol Biol Phys. 2006;66:319–20.
Sanghere P, Wong DW, McConkey CC, Geh JI, Hartley A. Chemoradiotherapy for rectal cancer: an updated analysis of factors affecting pathological response. Clin Oncol (R Coll Radiol). 2008;20:176–86.
Ang KK, Sturgis EM. Human papillomavirus as a marker of the natural history and response to therapy of head and neck squamous cell carcinoma. Semin Radiat Oncol. 2012;22:128–42.
Valentini V, van Stiphout R, Lammering G, Gambacorta MA, Barba MC, Bebenek M, et al. Nomograms for predicting local recurrence, distant metastases, and overall survival for patients with locally advanced rectal cancer on the basis of European randomized clinical trials. J Clin Oncol. 2011;29:3163–72.
Acknowledgments
This study was financed in part by a research grant from Mutua Madrileña Biomedical, Foundation Institute Health Research Marañón (study code CMF, FMM 06-02).
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 110 kb)
Rights and permissions
About this article
Cite this article
Sole, C.V., Calvo, F.A., Ferrer, C. et al. Human cytomegalovirus and Epstein-Barr virus infection impact on 18F-FDG PET/CT SUVmax, CT volumetric and KRAS-based parameters of patients with locally advanced rectal cancer treated with neoadjuvant therapy. Eur J Nucl Med Mol Imaging 42, 186–196 (2015). https://doi.org/10.1007/s00259-014-2910-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-014-2910-8