Abstract
Purpose
The purpose of this study was to investigate the prognostic value of 18F-fluorodeoxyglucose (FDG) positron emission tomography/computed tomography (PET/CT) in patients with ampullary adenocarcinoma (AAC) after curative surgical resection.
Methods
Fifty-two patients with AAC who had undergone 18F-FDG PET/CT and subsequent curative resections were retrospectively enrolled. The maximum standardized uptake value (SUVmax) and tumor to background ratio (TBR) were measured on 18F-FDG PET/CT in all patients. The prognostic significances of PET/CT parameters and clinicopathologic factors for recurrence-free survival (RFS) and overall survival (OS) were evaluated by univariate and multivariate analyses.
Results
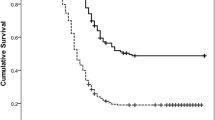
Of the 52 patients, 19 (36.5 %) experienced tumor recurrence during the follow-up period and 18 (35.8 %) died. The 3-year RFS and OS were 62.3 and 61.5 %, respectively. Preoperative CA19-9 level, tumor differentiation, presence of lymph node metastasis, SUVmax, and TBR were significant prognostic factors for both RFS and OS (p < 0.05) on univariate analyses, and patient age showed significance only for predicting RFS (p < 0.05). On multivariate analyses, SUVmax and TBR were independent prognostic factors for RFS, and tumor differentiation, SUVmax, and TBR were independent prognostic factors for OS.
Conclusion
SUVmax and TBR on preoperative 18F-FDG PET/CT are independent prognostic factors for predicting RFS and OS in patients with AAC; patients with high SUVmax (>4.80) or TBR (>1.75) had poor survival outcomes. The role of and indications for adjuvant therapy after curative resection of AAC are still unclear. 18F-FDG uptake in the primary tumor could provide additive prognostic information for the decision-making process regarding adjuvant therapy.


Similar content being viewed by others
References
Heinrich S, Clavien PA. Ampullary cancer. Curr Opin Gastroenterol 2010;26:280–5.
Howe JR, Klimstra DS, Moccia RD, Conlon KC, Brennan MF. Factors predictive of survival in ampullary carcinoma. Ann Surg 1998;228:87–94.
Choi SB, Kim WB, Song TJ, Suh SO, Kim YC, Choi SY. Surgical outcomes and prognostic factors for ampulla of Vater cancer. Scand J Surg 2011;100:92–8.
Romiti A, Barucca V, Zullo A, Sarcina I, Di Rocco R, D’Antonio C, et al. Tumors of ampulla of Vater: a case series and review of chemotherapy options. World J Gastrointest Oncol 2012;4:60–7.
Kim WS, Choi DW, Choi SH, Heo JS, You DD, Lee HG. Clinical significance of pathologic subtype in curatively resected ampulla of vater cancer. J Surg Oncol 2012;105:266–72.
Lowe MC, Coban I, Adsay NV, Sarmiento JM, Chu CK, Staley CA, et al. Important prognostic factors in adenocarcinoma of the ampulla of Vater. Am Surg 2009;75:754–60. discussion 761.
Robert PE, Leux C, Ouaissi M, Miguet M, Paye F, Merdrignac A, et al. Predictors of long-term survival following resection for ampullary carcinoma: a large retrospective French multicentric study. Pancreas 2014;43:692–7.
Woo SM, Ryu JK, Lee SH, Yoo JW, Park JK, Kim YT, et al. Recurrence and prognostic factors of ampullary carcinoma after radical resection: comparison with distal extrahepatic cholangiocarcinoma. Ann Surg Oncol 2007;14:3195–201.
Kurihara C, Yoshimi F, Sasaki K, Iijima T, Kawasaki H, Nagai H. Clinical value of serum CA19-9 as a prognostic factor for the ampulla of Vater carcinoma. Hepatogastroenterology. 2013;60:1588–91.
Chang DK, Jamieson NB, Johns AL, Scarlett CJ, Pajic M, Chou A, et al. Histomolecular phenotypes and outcome in adenocarcinoma of the ampulla of vater. J Clin Oncol 2013;31:1348–56.
Lee JH, Lee KG, Ha TK, Jun YJ, Paik SS, Park HK, et al. Pattern analysis of lymph node metastasis and the prognostic importance of number of metastatic nodes in ampullary adenocarcinoma. Am Surg 2011;77:322–9.
Cheng MF, Wang HP, Tien YW, Liu KL, Yen RF, Tzen KY, et al. Usefulness of PET/CT for the differentiation and characterization of periampullary lesions. Clin Nucl Med 2013;38:703–8.
Raj P, Kaman L, Singh R, Dahyia D, Bhattacharya A, Bal A. Sensitivity and specificity of FDG PET-CT scan in detecting lymph node metastasis in operable periampullary tumours in correlation with the final histopathology after curative surgery. Updates Surg 2013;65:103–7.
Sperti C, Pasquali C, Fiore V, Bissoli S, Chierichetti F, Liessi G, et al. Clinical usefulness of 18-fluorodeoxyglucose positron emission tomography in the management of patients with nonpancreatic periampullary neoplasms. Am J Surg 2006;191:743–8.
Choi HJ, Kang CM, Lee WJ, Song SY, Cho A, Yun M, et al. Prognostic value of 18F-fluorodeoxyglucose positron emission tomography in patients with resectable pancreatic cancer. Yonsei Med J 2013;54:1377–83.
Kitamura K, Hatano E, Higashi T, Seo S, Nakamoto Y, Narita M, et al. Prognostic value of (18)F-fluorodeoxyglucose positron emission tomography in patients with extrahepatic bile duct cancer. J Hepatobiliary Pancreat Sci 2011;18:39–46.
Furukawa H, Ikuma H, Asakura K, Uesaka K. Prognostic importance of standardized uptake value on F-18 fluorodeoxyglucose-positron emission tomography in biliary tract carcinoma. J Surg Oncol 2009;100:494–9.
Hwang J, Lim I, Chang K, Kim B, Choi C, Lim S. Prognostic value of SUVmax measured by fluorine-18 fluorodeoxyglucose positron emission tomography with computed tomography in patients with pancreatic cancer. Nucl Med Mol Imaging 2012;46:207–14.
Kalady MF, Clary BM, Clark LA, Gottfried M, Rohren EM, Coleman RE, et al. Clinical utility of positron emission tomography in the diagnosis and management of periampullary neoplasms. Ann Surg Oncol 2002;9:799–806.
Neoptolemos JP, Moore MJ, Cox TF, Valle JW, Palmer DH, McDonald AC, et al. Effect of adjuvant chemotherapy with fluorouracil plus folinic acid or gemcitabine vs observation on survival in patients with resected periampullary adenocarcinoma: the ESPAC-3 periampullary cancer randomized trial. JAMA 2012;308:147–56.
Kim K, Chie EK, Jang JY, Kim SW, Han SW, Oh DY, et al. Prognostic significance of tumour location after adjuvant chemoradiotherapy for periampullary adenocarcinoma. Clin Transl Oncol 2012;14:391–5.
Narang AK, Miller RC, Hsu CC, Bhatia S, Pawlik TM, Laheru D, et al. Evaluation of adjuvant chemoradiation therapy for ampullary adenocarcinoma: the Johns Hopkins Hospital-Mayo Clinic collaborative study. Radiat Oncol 2011;6:126.
Bhatia S, Miller RC, Haddock MG, Donohue JH, Krishnan S. Adjuvant therapy for ampullary carcinomas: the Mayo Clinic experience. Int J Radiat Oncol Biol Phys 2006;66:514–9.
Kim CK, Gupta NC, Chandramouli B, Alavi A. Standardized uptake values of FDG: body surface area correction is preferable to body weight correction. J Nucl Med 1994;35:164–7.
Zasadny KR, Wahl RL. Standardized uptake values of normal tissues at PET with 2-[fluorine-18]-fluoro-2-deoxy-D-glucose: variations with body weight and a method for correction. Radiology 1993;189:847–50.
Hamberg LM, Hunter GJ, Alpert NM, Choi NC, Babich JW, Fischman AJ. The dose uptake ratio as an index of glucose metabolism: useful parameter or oversimplification? J Nucl Med 1994;35:1308–12.
Lindholm P, Minn H, Leskinen-Kallio S, Bergman J, Ruotsalainen U, Joensuu H. Influence of the blood glucose concentration on FDG uptake in cancer–a PET study. J Nucl Med 1993;34:1–6.
Park KW, Ashlock R, Chang WB, Lahner J, Line B, Kim C. High variation in standardized uptake values among PET systems from different manufacturers. J Nucl Med 2007;48(Suppl 2):185P.
Morini S, Perrone G, Borzomati D, Vincenzi B, Rabitti C, Righi D, et al. Carcinoma of the ampulla of Vater: morphological and immunophenotypical classification predicts overall survival. Pancreas 2013;42:60–6.
Kim HS, Shin SJ, Kim JH, Kim H, Choi HJ. Better outcome of XELOX chemotherapy in patients with advanced intestinal-type adenocarcinoma of the ampulla of Vater. Tohoku J Exp Med 2013;231:21–8.
Park HH, Park DS, Kweon DC, Lee SB, Oh KB, Lee JD, et al. Inter-comparison of 18F-FDG PET/CT standardized uptake values in Korea. Appl Radiat Isot 2011;69:241–6.
Dholakia AS, Chaudhry M, Leal JP, Chang DT, Raman SP, Hacker-Prietz A, et al. Baseline metabolic tumor volume and total lesion glycolysis are associated with survival outcomes in patients with locally advanced pancreatic cancer receiving stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys 2014;89:539–46.
Lee JW, Kang CM, Choi HJ, Lee WJ, Song SY, Lee JH, et al. Prognostic value of metabolic tumor volume and total lesion glycolysis on preoperative 18F-FDG PET/CT in patients with pancreatic cancer. J Nucl Med 2014;55:898–904.
Haug AR, Heinemann V, Bruns CJ, Hoffmann R, Jakobs T, Bartenstein P, et al. 18F-FDG PET independently predicts survival in patients with cholangiocellular carcinoma treated with 90Y microspheres. Eur J Nucl Med Mol Imaging 2011;38:1037–45.
Acknowledgments
This study was supported by a new faculty research seed money grant of Yonsei University College of Medicine for 2014 (2014-32-0026).
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Choi, H.J., Kang, C.M., Jo, K. et al. Prognostic significance of standardized uptake value on preoperative 18F-FDG PET/CT in patients with ampullary adenocarcinoma. Eur J Nucl Med Mol Imaging 42, 841–847 (2015). https://doi.org/10.1007/s00259-014-2907-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-014-2907-3