Abstract
Purpose
Several lines of evidence strongly implicate a dysfunctional endocannabinoid system (ECS) in eating disorders. Using [18F]MK-9470 and small animal positron emission tomography (PET), we investigated for the first time cerebral changes in type 1 cannabinoid (CB1) receptor binding in vivo in the activity-based rat model of anorexia (ABA), in comparison to distinct motor- and food-related control conditions and in relation to gender and behavioural variables.
Methods
In total, experiments were conducted on 80 Wistar rats (23 male and 57 female). Male rats were assigned to the cross-sectional conditions: ABA (n = 12) and CONTROL (n = 11), whereas female rats were divided between two settings: (1) a cross-sectional design using ABA (n = 13), CONTROL (n = 9), and two extra control conditions for each of the variables manipulated in ABA, i.e. DIET (n = 8) and WHEEL (n = 9), and (2) a longitudinal one using ABA (n = 10) and CONTROL (n = 8) studied at baseline, during the model and upon recovery. The ABA group was subjected to food restriction in the presence of a running wheel, the DIET group to food restriction without wheel, the WHEEL group to a normal diet with wheel and CONTROL animals had a normal diet and no running wheel. Parametric CB1 receptor images of each group were spatially normalized to Paxinos space and analysed voxel-wise.
Results
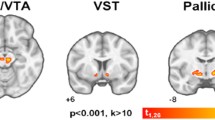
In the ABA model, absolute [18F]MK-9470 binding was significantly increased in all cortical and subcortical brain areas as compared to control conditions (male +67 %; female >51 %, all p cluster < 6.3×10−6) that normalized towards baseline values after weight gain. Additionally, relative [18F]MK-9470 binding was increased in the hippocampus, inferior colliculus and entorhinal cortex of female ABA (+4.6 %; p cluster < 1.3×10−6), whereas no regional differences were observed in male subjects. Again, relative [18F]MK-9470 binding values normalized upon weight gain.
Conclusion
These data point to a widespread transient disturbance of the endocannabinoid transmission, specifically for CB1 receptors in the ABA model. Our data also suggest (1) gender effects on regional CB1 receptor binding in the hippocampus and (2) add further proof to the validity of the ABA model to mimic aspects of human disease.







Similar content being viewed by others
References
Fairburn CG, Harrison PJ. Eating disorders. Lancet 2003;361:407–16.
American Psychiatric Association. Treatment of patients with eating disorders, third edition. American Psychiatric Association. Am J Psychiatry 2006; 163:4–54.
Katona I, Freund TF. Endocannabinoid signaling as a synaptic circuit breaker in neurological disease. Nat Med 2008;14:923–30.
Cota D, Steiner MA, Marsicano G, Cervino C, Herman JP, Grübler Y, et al. Requirement of cannabinoid receptor type 1 for the basal modulation of hypothalamic-pituitary-adrenal axis function. Endocrinology 2007;148:1574–81.
Solinas M, Yasar S, Goldberg SR. Endocannabinoid system involvement in brain reward processes related to drug abuse. Pharmacol Res 2007;56:393–405.
Wilson RI, Nicoll RA. Endocannabinoid signaling in the brain. Science 2002;296:678–82.
Di Marzo V, Bifulco M, De Petrocellis L. The endocannabinoid system and its therapeutic exploitation. Nat Rev Drug Discov 2004;3:771–84.
Monteleone P, Matias I, Martiadis V, De Petrocellis L, Maj M, Di Marzo V. Blood levels of the endocannabinoid anandamide are increased in anorexia nervosa and in binge-eating disorder, but not in bulimia nervosa. Neuropsychopharmacology 2005;30:1216–21.
Hanus L, Avraham Y, Ben-Shushan D, Zolotarev O, Berry EM, Mechoulam R. Short-term fasting and prolonged semistarvation have opposite effects on 2-AG levels in mouse brain. Brain Res 2003;983:144–51.
Wiley JL, Burston JJ, Leggett DC, Alekseeva OO, Razdan RK, Mahadevan A, et al. CB1 cannabinoid receptor-mediated modulation of food intake in mice. Br J Pharmacol 2005;145:293–300.
Siegfried Z, Kanyas K, Latzer Y, Karni O, Bloch M, Lerer B, et al. Association study of cannabinoid receptor gene (CNR1) alleles and anorexia nervosa: differences between restricting and binging/purging subtypes. Am J Med Genet B Neuropsychiatr Genet 2004;125B:126–30.
Monteleone P, Bifulco M, Di Filippo C, Gazzerro P, Canestrelli B, Monteleone F, et al. Association of CNR1 and FAAH endocannabinoid gene polymorphisms with anorexia nervosa and bulimia nervosa: evidence for synergistic effects. Genes Brain Behav 2009;8:728–32.
Beal JE, Olson R, Lefkowitz L, Laubenstein L, Bellman P, Yangco B, et al. Long-term efficacy and safety of dronabinol for acquired immunodeficiency syndrome-associated anorexia. J Pain Symptom Manage 1997;14:7–14.
Arias Horcajadas F. Cannabinoids in eating disorders and obesity. Mol Neurobiol 2007;36:113–28.
Gross H, Ebert MH, Faden VB, Goldberg SC, Kaye WH, Caine ED, et al. A double-blind trial of delta 9-tetrahydrocannabinol in primary anorexia nervosa. J Clin Psychopharmacol 1983;3:165–71.
Gérard N, Pieters G, Goffin K, Bormans G, Van Laere K. Brain type 1 cannabinoid receptor availability in patients with anorexia and bulimia nervosa. Biol Psychiatry 2011;70:777–84.
Rorato R, Reis WL, de Carvalho BB, Antunes-Rodrigues J, Elias LL. Cannabinoid CB(1) receptor restrains accentuated activity of hypothalamic corticotropin-releasing factor and brainstem tyrosine hydroxylase neurons in endotoxemia-induced hypophagia in rats. Neuropharmacology 2012;63:154–60.
Burns HD, Van Laere K, Sanabria-Bohórquez S, Hamill TG, Bormans G, Eng WS, et al. [18F]MK-9470, a positron emission tomography (PET) tracer for in vivo human PET brain imaging of the cannabinoid-1 receptor. Proc Natl Acad Sci U S A 2007;104:9800–5.
Hillebrand JJ, Koeners MP, de Rijke CE, Kas MJ, Adan RA. Leptin treatment in activity-based anorexia. Biol Psychiatry 2005;58:165–71.
Hillebrand JJ, Heinsbroek AC, Kas MJ, Adan RA. The appetite suppressant d-fenfluramine reduces water intake, but not food intake, in activity-based anorexia. J Mol Endocrinol 2006;36:153–62.
Dixon DP, Ackert AM, Eckel LA. Development of, and recovery from, activity-based anorexia in female rats. Physiol Behav 2003;80:273–9.
Barbarich-Marsteller NC, Marsteller DA, Alexoff DL, Fowler JS, Dewey SL. MicroPET imaging in an animal model of anorexia nervosa. Synapse 2005;57:85–90.
van Kuyck K, Casteels C, Vermaelen P, Bormans G, Nuttin B, Van Laere K. Motor- and food-related metabolic cerebral changes in the activity-based rat model for anorexia nervosa: a voxel-based microPET study. Neuroimage 2007;35:214–21.
Marcondes FK, Bianchi FJ, Tanno AP. Determination of the estrous cycle phases of rats: some helpful considerations. Braz J Biol 2002;62:609–14.
Casteels C, Koole M, Celen S, Bormans G, Van Laere K. Preclinical evaluation and quantification of [(18)F]MK-9470 as a radioligand for PET imaging of the type 1 cannabinoid receptor in rat brain. Eur J Nucl Med Mol Imaging 2012;39:1467–77.
Casteels C, Bormans G, Van Laere K. The effect of anaesthesia on [(18)F]MK-9470 binding to the type 1 cannabinoid receptor in the rat brain. Eur J Nucl Med Mol Imaging 2010;37:1164–73.
Boellaard R, van Lingen A, Lammertsma AA. Experimental and clinical evaluation of iterative reconstruction (OSEM) in dynamic PET: quantitative characteristics and effects on kinetic modeling. J Nucl Med 2001;42:808–17.
Hoekzema E, Rojas S, Herance R, Pareto D, Abad S, Jiménez X, et al. [(11)C]-DASB microPET imaging in the aged rat: frontal and meso-thalamic increases in serotonin transporter binding. Exp Gerontol 2011;46:1020–5.
Thie JA, Hubner KF, Isidoro FP, Smith GT. A weight index for the standardized uptake value in 2-deoxy-2-[F-18]fluoro-D-glucose-positron emission tomography. Mol Imaging Biol 2007;9:91–8.
Sanabria-Bohórquez SM, Hamill TG, Goffin K, De Lepeleire I, Bormans G, Burns HD, et al. Kinetic analysis of the cannabinoid-1 receptor PET tracer [(18)F]MK-9470 in human brain. Eur J Nucl Med Mol Imaging 2010;37:920–33.
Goffin K, Bormans G, Casteels C, Bosier B, Lambert DM, Grachev ID, et al. An in vivo [(18)F]MK-9470 microPET study of type 1 cannabinoid receptor binding in Wistar rats after chronic administration of valproate and levetiracetam. Neuropharmacology 2008;54:1103–6.
Casteels C, Vermaelen P, Nuyts J, Van Der Linden A, Baekelandt V, Mortelmans L, et al. Construction and evaluation of multitracer small-animal PET probabilistic atlases for voxel-based functional mapping of the rat brain. J Nucl Med 2006;47:1858–66.
Casteels C, Vandeputte C, Rangarajan JR, Dresselaers T, Riess O, Bormans G, et al. Metabolic and type 1 cannabinoid receptor imaging of a transgenic rat model in the early phase of Huntington disease. Exp Neurol 2011;229:440–9.
Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, et al. Cannabinoid receptor localization in brain. Proc Natl Acad Sci U S A 1990;87:1932–6.
Matias I, Di Marzo V. Endocannabinoids and the control of energy balance. Trends Endocrinol Metab 2007;18:27–37.
Solinas M, Goldberg SR, Piomelli D. The endocannabinoid system in brain reward processes. Br J Pharmacol 2008;154:369–83.
Di Marzo V, Ligresti A, Cristino L. The endocannabinoid system as a link between homoeostatic and hedonic pathways involved in energy balance regulation. Int J Obes (Lond) 2009;33 Suppl 2:S18–24.
Di Marzo V, Goparaju SK, Wang L, Liu J, Bátkai S, Járai Z, et al. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature 2001;410:822–5.
McLaughlin PJ, Winston K, Swezey L, Wisniecki A, Aberman J, Tardif DJ, et al. The cannabinoid CB1 antagonists SR 141716A and AM 251 suppress food intake and food-reinforced behavior in a variety of tasks in rats. Behav Pharmacol 2003;14:583–8.
Rowland NE, Mukherjee M, Robertson K. Effects of the cannabinoid receptor antagonist SR 141716, alone and in combination with dexfenfluramine or naloxone, on food intake in rats. Psychopharmacology (Berl) 2001;159:111–6.
De Vry J, Schreiber R, Eckel G, Jentzsch KR. Behavioral mechanisms underlying inhibition of food-maintained responding by the cannabinoid receptor antagonist/inverse agonist SR141716A. Eur J Pharmacol 2004;483:55–63.
Ravinet Trillou C, Delgorge C, Menet C, Arnone M, Soubrié P. CB1 cannabinoid receptor knockout in mice leads to leanness, resistance to diet-induced obesity and enhanced leptin sensitivity. Int J Obes Relat Metab Disord 2004;28:640–8.
Fride E, Bregman T, Kirkham TC. Endocannabinoids and food intake: newborn suckling and appetite regulation in adulthood. Exp Biol Med (Maywood) 2005;230:225–34.
Riad M, Zimmer L, Rbah L, Watkins KC, Hamon M, Descarries L. Acute treatment with the antidepressant fluoxetine internalizes 5-HT1A autoreceptors and reduces the in vivo binding of the PET radioligand [18F]MPPF in the nucleus raphe dorsalis of rat. J Neurosci 2004;24:5420–6.
Romero J, García L, Fernández-Ruiz JJ, Cebeira M, Ramos JA. Changes in rat brain cannabinoid binding sites after acute or chronic exposure to their endogenous agonist, anandamide, or to delta 9-tetrahydrocannabinol. Pharmacol Biochem Behav 1995;51:731–7.
Hill MN, Patel S, Carrier EJ, Rademacher DJ, Ormerod BK, Hillard CJ, et al. Downregulation of endocannabinoid signaling in the hippocampus following chronic unpredictable stress. Neuropsychopharmacology 2005;30:508–15.
Gifford AN, Bruneus M, Gatley SJ, Lan R, Makriyannis A, Volkow ND. Large receptor reserve for cannabinoid actions in the central nervous system. J Pharmacol Exp Ther 1999;288:478–83.
Barbarich-Marsteller NC, Fornal CA, Takase LF, Bocarsly ME, Arner C, Walsh BT, et al. Activity-based anorexia is associated with reduced hippocampal cell proliferation in adolescent female rats. Behav Brain Res 2013;236:251–7.
Hill MN, Titterness AK, Morrish AC, Carrier EJ, Lee TT, Gil-Mohapel J, et al. Endogenous cannabinoid signaling is required for voluntary exercise-induced enhancement of progenitor cell proliferation in the hippocampus. Hippocampus 2010;20:513–23.
Aoki C, Sabaliauskas N, Chowdhury T, Min JY, Colacino AR, Laurino K, et al. Adolescent female rats exhibiting activity-based anorexia express elevated levels of GABA(A) receptor alpha4 and delta subunits at the plasma membrane of hippocampal CA1 spines. Synapse 2012;66:391–407.
Schenberg LC, Póvoa RM, Costa AL, Caldellas AV, Tufik S, Bittencourt AS. Functional specializations within the tectum defense systems of the rat. Neurosci Biobehav Rev 2005;29:1279–98.
Coddington E, Lewis C, Rose JD, Moore FL. Endocannabinoids mediate the effects of acute stress and corticosterone on sex behavior. Endocrinology 2007;148:493–500.
Mani SK, Mitchell A, O’Malley BW. Progesterone receptor and dopamine receptors are required in delta 9-tetrahydrocannabinol modulation of sexual receptivity in female rats. Proc Natl Acad Sci U S A 2001;98:1249–54.
Acknowledgments
Merck & Co, Inc. is acknowledged for the availability of the [18F]MK-9470 precursor, and for their critical revision of this manuscript and their suggestions for improvement. The authors thank Peter Vermaelen and Ann Van Santvoort for their assistance in data acquisition, as well as the Leuven PET radiopharmacy team for tracer preparations. Financial support of the Research Council of the Katholieke Universiteit Leuven (OT/05/58), the Fund for Scientific Research, Flanders, Belgium (FWO/G.0548.06), the K.U. Leuven Molecular Small Animal Imaging Center (KUL EF/05/08) is gratefully acknowledged. Cindy Casteels and Kris van Kuyck are supported by a postdoctoral mandate of the Flemish Fund for Scientific Research. Koen Van Laere is a senior clinical investigator of the Flemish Fund for Scientific Research.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
Brain and plasma kinetics of [18F]MK-9470 in ABA. Average tissue-activity curves in the cortex (a) and average metabolite-corrected plasma input curves (b) of ABA (○) and CONTROL (□) rats (n = 2/group). Insert: detail of curves in b between 0 and 600 min. Data are given as mean ± SD. (TIFF 552 kb) (JPEG 12 kb)
Rights and permissions
About this article
Cite this article
Casteels, C., Gérard, N., van Kuyck, K. et al. Small animal PET imaging of the type 1 cannabinoid receptor in a rodent model for anorexia nervosa. Eur J Nucl Med Mol Imaging 41, 308–321 (2014). https://doi.org/10.1007/s00259-013-2522-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-013-2522-8