Abstract
Purpose
Among patients with advanced non-small cell lung cancer (NSCLC), identification of a subgroup of patients for immediate maintenance treatment after first-line chemotherapy has great importance in improving survival. The purpose of this study was to investigate whether the metabolic responses evaluated by 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) may be a potential screening tool for identifying patients with early disease progression who may benefit from immediate maintenance treatment.
Methods
A total of 52 patients with advanced NSCLC (36 men and 16 women, mean age 57.2 ± 10.6 years) who underwent baseline and follow-up 18F-FDG PET/CT after four cycles of first-line chemotherapy were enrolled. Maximum standardized uptake value (SUVmax), SUVpeak, metabolic tumour volume (MTV) and total lesion glycolysis (TLG) of the tumour lesions were measured and percentage decrease of the parameters was calculated. The prognostic significance of percentage decrease of these parameters and other clinical variables related to progression-free survival (PFS) and overall survival (OS) were assessed by Cox proportional hazards regression analysis. Receiver-operating characteristic (ROC) curve analysis was used to define the optimal cut-off value of percentage decrease of the parameters that could distinguish between early (PFS < 6 months) and late (PFS ≥ 6 months) disease progression groups.
Results
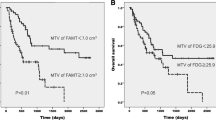
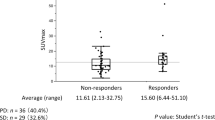
Multivariate analysis showed that percentage decrease of TLG [hazard ratio per 10 % decrease = 1.030, 95 % confidence interval (CI) = 1.012–1.048, p = 0.001) was a significant predictor of PFS and OS. ROC curves identified a 50.0 % decrease in TLG as the optimal cut-off value to distinguish disease progression groups. Positive and negative predictive values of the optimal TLG value for selecting patients with late disease progression were 36.4 and 100.0 %, respectively.
Conclusion
The percentage decrease in TLG of measurable tumour lesions may be a potential parameter to appropriately identify a subgroup of patients for immediate maintenance treatment after first-line chemotherapy in patients with advanced NSCLC.


Similar content being viewed by others
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011;61:69–90.
Azzoli CG, Baker Jr S, Temin S, Pao W, Aliff T, Brahmer J, et al. American Society of Clinical Oncology Clinical Practice Guideline update on chemotherapy for stage IV non-small-cell lung cancer. J Clin Oncol 2009;27:6251–66.
Azzoli CG, Temin S, Aliff T, Baker Jr S, Brahmer J, Johnson DH, et al. 2011 Focused update of 2009 American Society of Clinical Oncology Clinical Practice Guideline update on chemotherapy for stage IV non-small-cell lung cancer. J Clin Oncol 2011;29:3825–31.
Schiller JH, Ramalingam SS. Duration of chemotherapy for metastatic non-small-cell lung cancer: more may be better after all. J Clin Oncol 2009;27:3265–7.
Ciuleanu T, Brodowicz T, Zielinski C, Kim JH, Krzakowski M, Laack E, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet 2009;374:1432–40.
Cappuzzo F, Ciuleanu T, Stelmakh L, Cicenas S, Szczésna A, Juhász E, et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol 2010;11:521–9.
Fidias PM, Dakhil SR, Lyss AP, Loesch DM, Waterhouse DM, Bromund JL, et al. Phase III study of immediate compared with delayed docetaxel after front-line therapy with gemcitabine plus carboplatin in advanced non-small-cell lung cancer. J Clin Oncol 2009;27:591–8.
Paz-Ares L, de Marinis F, Dediu M, Thomas M, Pujol JL, Bidoli P, et al. Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non-squamous non-small-cell lung cancer (PARAMOUNT): a double-blind, phase 3, randomised controlled trial. Lancet Oncol 2012;13:247–55.
Belani CP, Barstis J, Perry MC, La Rocca RV, Nattam SR, Rinaldi D, et al. Multicenter, randomized trial for stage IIIB or IV non-small-cell lung cancer using weekly paclitaxel and carboplatin followed by maintenance weekly paclitaxel or observation. J Clin Oncol 2003;21:2933–9.
Gatzemeier U, Pluzanska A, Szczesna A, Kaukel E, Roubec J, De Rosa F, et al. Phase III study of erlotinib in combination with cisplatin and gemcitabine in advanced non-small-cell lung cancer: the Tarceva Lung Cancer Investigation Trial. J Clin Oncol 2007;25:1545–52.
Johnson EA, Marks RS, Mandrekar SJ, Hillman SL, Hauge MD, Bauman MD, et al. Phase III randomized, double-blind study of maintenance CAI or placebo in patients with advanced non-small cell lung cancer (NSCLC) after completion of initial therapy (NCCTG 97-24-51). Lung Cancer 2008;60:200–7.
Westeel V, Quoix E, Moro-Sibilot D, Mercier M, Breton JL, Debieuvre D, et al. Randomized study of maintenance vinorelbine in responders with advanced non-small-cell lung cancer. J Natl Cancer Inst 2005;97:499–506.
Leighl NB, Paz-Ares L, Douillard JY, Peschel C, Arnold A, Depierre A, et al. Randomized phase III study of matrix metalloproteinase inhibitor BMS-275291 in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: National Cancer Institute of Canada-Clinical Trials Group Study BR.18. J Clin Oncol 2005;23:2831–9.
Brodowicz T, Krzakowski M, Zwitter M, Tzekova V, Ramlau R, Ghilezan N, et al. Cisplatin and gemcitabine first-line chemotherapy followed by maintenance gemcitabine or best supportive care in advanced non-small cell lung cancer: a phase III trial. Lung Cancer 2006;52:155–63.
Yoon DH, Baek S, Choi CM, Lee DH, Suh C, Ryu JS, et al. FDG-PET as a potential tool for selecting patients with advanced non-small cell lung cancer who may be spared maintenance therapy after first-line chemotherapy. Clin Cancer Res 2011;17:5093–100.
Hicks RJ. Role of 18F-FDG PET in assessment of response in non-small cell lung cancer. J Nucl Med 2009;50 Suppl 1:31S–42.
Nahmias C, Hanna WT, Wahl LM, Long MJ, Hubner KF, Townsend DW. Time course of early response to chemotherapy in non-small cell lung cancer patients with 18F-FDG PET/CT. J Nucl Med 2007;48:744–51.
Berghmans T, Dusart M, Paesmans M, Hossein-Foucher C, Buvat I, Castaigne C, et al. Primary tumor standardized uptake value (SUVmax) measured on fluorodeoxyglucose positron emission tomography (FDG-PET) is of prognostic value for survival in non-small cell lung cancer (NSCLC): a systematic review and meta-analysis (MA) by the European Lung Cancer Working Party for the IASLC Lung Cancer Staging Project. J Thorac Oncol 2008;3:6–12.
Hyun SH, Choi JY, Shim YM, Kim K, Lee SJ, Cho YS, et al. Prognostic value of metabolic tumor volume measured by 18F-fluorodeoxyglucose positron emission tomography in patients with esophageal carcinoma. Ann Surg Oncol 2010;17:115–22.
Moon SH, Choi JY, Lee HJ, Son YI, Baek CH, Ahn YC, et al. Prognostic value of 18F-FDG PET/CT in patients with squamous cell carcinoma of the tonsil: comparisons of volume-based metabolic parameters. Head Neck 2013;35:15–22. doi: 10.1002/hed.22904.
Choi K, Yoo I, Han E, Kim Y, Kim GW, Na SJ, et al. Prognostic value of metabolic tumor volume measured by 18F-FDG PET/CT in locally advanced head and neck squamous cell carcinomas treated by surgery. Nucl Med Mol Imaging 2011;45:43–51.
Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med 2009;50 Suppl 1:122S–150.
Juweid ME, Stroobants S, Hoekstra OS, Mottaghy FM, Dietlein M, Guermazi A, et al. Use of positron emission tomography for response assessment of lymphoma: consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. J Clin Oncol 2007;25:571–8.
Pfister DG, Johnson DH, Azzoli CG, Sause W, Smith TJ, Baker Jr S, et al. American Society of Clinical Oncology treatment of unresectable non-small-cell lung cancer guideline: update 2003. J Clin Oncol 2004;22:330–53.
Pirker R, Pereira JR, Szczesna A, von Pawel J, Krzakowski M, Ramlau R, et al. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): an open-label randomised phase III trial. Lancet 2009;373:1525–31.
Park JO, Kim SW, Ahn JS, Suh C, Lee JS, Jang JS, et al. Phase III trial of two versus four additional cycles in patients who are nonprogressive after two cycles of platinum-based chemotherapy in non small-cell lung cancer. J Clin Oncol 2007;25:5233–9.
von Plessen C, Bergman B, Andresen O, Bremnes RM, Sundstrom S, Gilleryd M, et al. Palliative chemotherapy beyond three courses conveys no survival or consistent quality-of-life benefits in advanced non-small-cell lung cancer. Br J Cancer 2006;95:966–73.
Weber WA, Wieder H. Monitoring chemotherapy and radiotherapy of solid tumors. Eur J Nucl Med Mol Imaging 2006;33 Suppl 1:27–37.
Young H, Baum R, Cremerius U, Herholz K, Hoekstra O, Lammertsma AA, et al. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur J Cancer 1999;35:1773–82.
Lucignani G. Monitoring cancer therapy with PET: probably effective, but more research is needed. Eur J Nucl Med Mol Imaging 2009;36:1520–5.
Soret M, Bacharach SL, Buvat I. Partial-volume effect in PET tumor imaging. J Nucl Med 2007;48:932–45.
Lee HY, Hyun SH, Lee KS, Kim BT, Kim J, Shim YM, et al. Volume-based parameter of 18F-FDG PET/CT in malignant pleural mesothelioma: prediction of therapeutic response and prognostic implications. Ann Surg Oncol 2010;17:2787–94.
Hatt M, Visvikis D, Albarghach NM, Tixier F, Pradier O, Cheze-le Rest C. Prognostic value of 18F-FDG PET image-based parameters in oesophageal cancer and impact of tumour delineation methodology. Eur J Nucl Med Mol Imaging 2011;38:1191–202.
Acknowledgments
This study was supported by a grant from the National R&D Program for Cancer Control, Ministry of Health and Welfare, Republic of Korea (#1120150).
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Seung Hwan Moon and Su-Hee Cho are co-first authors and contributed equally to this work.
Rights and permissions
About this article
Cite this article
Moon, S.H., Cho, SH., Park, L.C. et al. Metabolic response evaluated by 18F-FDG PET/CT as a potential screening tool in identifying a subgroup of patients with advanced non-small cell lung cancer for immediate maintenance therapy after first-line chemotherapy. Eur J Nucl Med Mol Imaging 40, 1005–1013 (2013). https://doi.org/10.1007/s00259-013-2400-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-013-2400-4