Abstract
Purpose
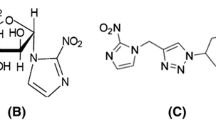
Tumour hypoxia is linked to treatment resistance. Positron emission tomography (PET) using hypoxia tracers such as fluoroazomycin arabinoside (FAZA) may allow identification of patients with hypoxic tumours and the monitoring of the efficacy of hypoxia-targeting treatment. Since hypoxia PET is characterized by poor image contrast, and tumour hypoxia undergoes spontaneous changes and is affected by therapy, it remains unclear to what extent PET scans are reproducible. Tumour-bearing mice are valuable in the validation of hypoxia PET, but identification of a reliable reference tissue value (blood sample or image-derived muscle value) for repeated scans may be difficult due to the small size of the animal or absence of anatomical information (pure PET). Here tumour hypoxia was monitored over time using repeated PET scans in individual tumour-bearing mice before and during fractionated radiotherapy.
Methods
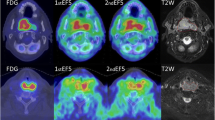
Mice bearing human SiHa cervix tumour xenografts underwent a PET scan 3 h following injection of FAZA on two consecutive days before initiation of treatment (baseline) and again following irradiation with four and ten fractions of 2.5 Gy. On the last scan day, mice were given an intraperitoneal injection of pimonidazole (hypoxia marker), tumours were collected and the intratumoral distribution of FAZA (autoradiography) and hypoxia (pimonidazole immunohistology) were determined in cryosections.
Results
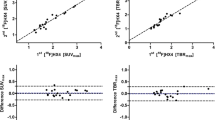
Tissue section analysis revealed that the intratumoral distribution of FAZA was strongly correlated with the regional density of hypoxic (pimonidazole-positive) cells, even when necrosis was present, suggesting that FAZA PET provides a reliable measure of tumour hypoxia at the time of the scan. PET-based quantification of tumour tracer uptake relative to injected dose showed excellent reproducibility at baseline, whereas normalization using an image-derived nonhypoxic reference tissue (muscle) proved highly unreliable since a valid and reliable reference value could not be determined. The intratumoral distribution of tracer was stable at baseline as shown by a voxel-by-voxel comparison of the two scans (R = 0.82, range 0.72–0.90). During treatment, overall tracer retention changed in individual mice, but there was no evidence of general reoxygenation.
Conclusion
Hypoxia PET scans are quantitatively correct and highly reproducible in tumour-bearing mice. Preclinical hypoxia PET is therefore a valuable and reliable tool for the development of strategies that target or modify hypoxia.






Similar content being viewed by others
References
Vaupel P, Harrison L. Tumor hypoxia: causative factors, compensatory mechanisms, and cellular response. Oncologist. 2004;9 Suppl 5:4–9.
Olive PL. Radiation-induced reoxygenation in the SCCVII murine tumour: evidence for a decrease in oxygen consumption and an increase in tumour perfusion. Radiother Oncol. 1994;32:37–46.
Crokart N, Jordan BF, Baudelet C, et al. Early reoxygenation in tumors after irradiation: determining factors and consequences for radiotherapy regimens using daily multiple fractions. Int J Radiat Oncol Biol Phys. 2005;63:901–10.
Dunst J, Hansgen G, Lautenschlager C, Fuchsel G, Becker A. Oxygenation of cervical cancers during radiotherapy and radiotherapy + cis-retinoic acid/interferon. Int J Radiat Oncol Biol Phys. 1999;43:367–73.
Harriss W, Bezak E, Yeoh E, Hermans M. Measurement of reoxygenation during fractionated radiotherapy in head and neck squamous cell carcinoma xenografts. Australas Phys Eng Sci Med. 2010;33:251–63.
Stadler P, Feldmann HJ, Creighton C, Kau R, Molls M. Changes in tumor oxygenation during combined treatment with split-course radiotherapy and chemotherapy in patients with head and neck cancer. Radiother Oncol. 1998;48:157–64.
Santiago A, Eicheler W, Bussink J, et al. Effect of cetuximab and fractionated irradiation on tumour micro-environment. Radiother Oncol. 2010;97:322–9.
Yaromina A, Kroeber T, Meinzer A, et al. Exploratory study of the prognostic value of microenvironmental parameters during fractionated irradiation in human squamous cell carcinoma xenografts. Int J Radiat Oncol Biol Phys. 2011;80:1205–13.
Eschmann SM, Paulsen F, Bedeshem C, et al. Hypoxia-imaging with (18)F-misonidazole and PET: changes of kinetics during radiotherapy of head-and-neck cancer. Radiother Oncol. 2007;83:406–10.
Achermann RE, Ohlerth SM, Rohrer BC, et al. Oxygenation of spontaneous canine tumors during fractionated radiation therapy. Strahlenther Onkol. 2004;180:297–305.
Thames Jr HD, Peters LJ, Withers HR, Fletcher GH. Accelerated fractionation vs hyperfractionation: rationales for several treatments per day. Int J Radiat Oncol Biol Phys. 1983;9:127–38.
Horsman MR, Overgaard J. Preclinical studies on how to deal with patient intolerance to nicotinamide and carbogen. Radiother Oncol. 2004;70:301–9.
Janssens GO, Rademakers SE, Terhaard CH, et al. Accelerated radiotherapy with carbogen and nicotinamide for laryngeal cancer: results of a phase III randomized trial. J Clin Oncol. 2012;30:1777–83.
Overgaard J, Horsman MR. Modification of hypoxia-induced radioresistance in tumors by the use of oxygen and sensitizers. Semin Radiat Oncol. 1996;6:10–21.
Overgaard J. Hypoxic radiosensitization: adored and ignored. J Clin Oncol. 2007;25:4066–74.
Beck R, Roper B, Carlsen JM, et al. Pretreatment 18F-FAZA PET predicts success of hypoxia-directed radiochemotherapy using tirapazamine. J Nucl Med. 2007;48:973–80.
Reddy SB, Williamson SK. Tirapazamine: a novel agent targeting hypoxic tumor cells. Expert Opin Investig Drugs. 2009;18:77–87.
Thorwarth D, Eschmann SM, Paulsen F, Alber M. Hypoxia dose painting by numbers: a planning study. Int J Radiat Oncol Biol Phys. 2007;68:291–300.
Toustrup K, Sorensen BS, Nordsmark M, et al. Development of a hypoxia gene expression classifier with predictive impact for hypoxic modification of radiotherapy in head and neck cancer. Cancer Res. 2011;71:5923–31.
Nehmeh SA, Lee NY, Schroder H, et al. Reproducibility of intratumor distribution of (18)F-fluoromisonidazole in head and neck cancer. Int J Radiat Oncol Biol Phys. 2008;70:235–42.
Lin Z, Mechalakos J, Nehmeh S, et al. The influence of changes in tumor hypoxia on dose-painting treatment plans based on 18F-FMISO positron emission tomography. Int J Radiat Oncol Biol Phys. 2008;70:1219–28.
Mortensen LS, Busk M, Nordsmark M, et al. Accessing radiation response using hypoxia PET imaging and oxygen sensitive electrodes: a preclinical study. Radiother Oncol. 2011;99:418–23.
Busk M, Horsman MR, Jakobsen S, et al. Imaging hypoxia in xenografted and murine tumors with 18F-fluoroazomycin arabinoside: a comparative study involving microPET, autoradiography, PO2-polarography, and fluorescence microscopy. Int J Radiat Oncol Biol Phys. 2008;70:1202–12.
Busk M, Walenta S, Mueller-Klieser W, et al. Inhibition of tumor lactate oxidation: consequences for the tumor microenvironment. Radiother Oncol. 2011;99:404–11.
Dewhirst MW, Kimura H, Rehmus SW, et al. Microvascular studies on the origins of perfusion-limited hypoxia. Br J Cancer Suppl. 1996;27:S247–51.
Lanzen J, Braun RD, Klitzman B, Brizel D, Secomb TW, Dewhirst MW. Direct demonstration of instabilities in oxygen concentrations within the extravascular compartment of an experimental tumor. Cancer Res. 2006;66:2219–23.
Baudelet C, Ansiaux R, Jordan BF, Havaux X, Macq B, Gallez B. Physiological noise in murine solid tumours using T2*-weighted gradient-echo imaging: a marker of tumour acute hypoxia? Phys Med Biol. 2004;49:3389–411.
Baudelet C, Cron GO, Ansiaux R, et al. The role of vessel maturation and vessel functionality in spontaneous fluctuations of T2*-weighted GRE signal within tumors. NMR Biomed. 2006;19:69–76.
Brurberg KG, Benjaminsen IC, Dorum LM, Rofstad EK. Fluctuations in tumor blood perfusion assessed by dynamic contrast-enhanced MRI. Magn Reson Med. 2007;58:473–81.
Magat J, Jordan BF, Cron GO, Gallez B. Noninvasive mapping of spontaneous fluctuations in tumor oxygenation using 19F MRI. Med Phys. 2010;37:5434–41.
Chen L, Zhang Z, Kolb HC, Walsh JC, Zhang J, Guan Y. 18F-HX4 hypoxia imaging with PET/CT in head and neck cancer: a comparison with 18F-FMISO. Nucl Med Commun. 2012;33:1096–1102.
Busk M, Horsman MR, Jakobsen S, et al. Can hypoxia-PET map hypoxic cell density heterogeneity accurately in an animal tumor model at a clinically obtainable image contrast? Radiother Oncol. 2009;92:429–36.
Busk M, Munk OL, Jakobsen S, et al. Assessing hypoxia in animal tumor models based on pharmacokinetic analysis of dynamic FAZA PET. Acta Oncol. 2010;49:922–33.
Tran LB, Bol A, Labar D, et al. Hypoxia imaging with the nitroimidazole (18)F-FAZA PET tracer: a comparison with OxyLite, EPR oximetry and (19)F-MRI relaxometry. Radiother Oncol 2012. doi:10.1016/j.radonc.2012.04.011
Tseng JR, Dandekar M, Subbarayan M, et al. Reproducibility of 3′-deoxy-3′-(18)F-fluorothymidine microPET studies in tumor xenografts in mice. J Nucl Med. 2005;46:1851–7.
Dandekar M, Tseng JR, Gambhir SS. Reproducibility of 18F-FDG microPET studies in mouse tumor xenografts. J Nucl Med. 2007;48:602–7.
Chaplin DJ, Trotter MJ, Skov KA, Horsman MR. Modification of tumour radiation response in vivo by the benzamide analogue pyrazinamide. Br J Cancer. 1990;62:561–6.
Horsman MR, Chaplin DJ, Overgaard J. Combination of nicotinamide and hyperthermia to eliminate radioresistant chronically and acutely hypoxic tumor cells. Cancer Res. 1990;50:7430–6.
Wang K, Yorke E, Nehmeh SA, Humm JL, Ling CC. Modeling acute and chronic hypoxia using serial images of 18F-FMISO PET. Med Phys. 2009;36:4400–8.
Acknowledgments
We thank Ms. M. Simonsen of the PET centre (Aarhus University Hospital), Ms. I.M. Horsman, Mr. M. Johannsen, Ms. D. Grand, Ms. P Schjerrbeck, Ms. M.V. Bjerre and Ms. M. Kristiansen of the Department of Experimental and Clinical Oncology (Aarhus University Hospital), and Lene H. Skjærris of the Institute of Pathology (Aarhus University Hospital) for excellent technical and practical assistance.
Financial support
This study was supported by EC FP7 (METOXIA) funding, by CIRRO—The Lundbeck Foundation Center for Interventional Research in Radiation Oncology and The Danish Cancer Society (Grant number R40-A2022-11-S2).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Busk, M., Mortensen, L.S., Nordsmark, M. et al. PET hypoxia imaging with FAZA: reproducibility at baseline and during fractionated radiotherapy in tumour-bearing mice. Eur J Nucl Med Mol Imaging 40, 186–197 (2013). https://doi.org/10.1007/s00259-012-2258-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-012-2258-x