Abstract
Purpose
The standardized added metabolic activity (SAM) is a new marker of total lesion glycolysis that avoids partial volume effect (PVE) and thresholding. SAM is calculated by drawing a volume of interest (VOI1) around the tumour and a larger VOI (VOI2) around VOI1. Subtracting the background activity in VOI2-VOI1 from VOI1 yields SAM. If VOI1 is set at a reasonable distance from the tumour, PVE are avoided. Phantom and initial clinical validation data are presented.
Methods
Spheres of a Jaszczak phantom were filled with a 5.4, 3.64 and 2.0 times higher concentration relative to background activity and positron emission tomography (PET) data were acquired during 10 min. SAM of all spheres was expressed as a percentage of the expected value (the actual activity ratio minus 1). In 15 patients a 10-min list-mode acquisition PET study centred on their primary squamous cell carcinoma (PSCC) was performed and images of 1-10 min reconstructed. SAM1-9min values of PSCC were expressed as a percentage of SAM10min. Nineteen patients suffering from liver metastases treated with chemotherapy underwent PET/CT prior to (scan 1) and after 3–6 cycles of chemotherapy (scan 2). SAM and maximum standardized uptake values (SUVmax) of the liver lesions on scan 1 (SAM1 and SUVmax1) and the percentage reduction between both ΔSAM and ΔSUVmax were related to Response Evaluation Criteria in Solid Tumors (RECIST) response.
Results
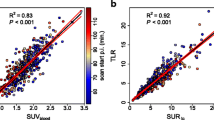
For the phantom acquisitions, the mean normalized SAM/sphere volume calculated was 94.9 % (SD 5.9 %) of the expected value. In the PSCC patients, the mean difference between SAM1min and SAM10min was only 4 % (SD 5 %). SUVmax1min and SUVmax10min proved to be not significantly different, but the variability was slightly larger than that of SAM (SD 6.4 %). SAM1 and ΔSAM values for responders versus non-responders were, respectively, 57 (SD 119) versus 297 (SD 625) for SAM1 (p = 0.2) and 99 % (SD 3 %) versus 32 % (SD 44 %) for ΔSAM (p = 0.001). SUVmax1 and ΔSUVmax values in responders versus non-responders were, respectively, 3.9 (SD 2.4) versus 6.3 (SD 3.1) for SUVmax1 (p = 0.08) and 94 % (SD 17) versus 7 % (SD 40 %) for ΔSUVmax (p = 0.0001). The AUC of ΔSAM and ΔSUVmax were not significantly different on receiver-operating characteristic (ROC) analysis (AUC 1.0 and 0.99, respectively, p = 0.6).
Conclusion
SAM is a promising parameter for tumour response assessment of liver metastases by means of 18F-fluorodeoxyglucose PET.


Similar content being viewed by others
References
Weber W, Figlin R. Monitoring cancer treatment with PET/CT: does it make a difference? J Nucl Med 2007;48:36S–44S.
Larson S, Erdi Y, Akhurst T, Mazumdar M, Macapinlac HA, Finn RD, et al. Tumor treatment response based on visual and quantitative changes in global tumor glycolysis using PET-FDG imaging. The visual response score and the change in total lesion glycolysis. Clin Positron Imaging 1999;2:159–71.
Zaidi H, El Naqa IE. PET-guided delineation of radiation therapy treatment volumes: a survey of image segmentation techniques. Eur J Nucl Med Mol Imaging 2010;37:2165–87.
Tylski P, Stute S, Grotus N, Doyeux K, Hapdey S, Gardin I, et al. Comparative assessment of methods for estimating tumor volume and standardized uptake value in (18)F-FDG PET. J Nucl Med 2010;51:268–76.
Weber WA. Quantitative analysis of PET studies. Radiother Oncol 2010;96:308–10.
Soret M, Bacharach S, Buvat I. Partial-volume effect in PET tumor imaging. J Nucl Med 2007;48:932–45.
Hoetjes N, van Velden F, Hoekstra O, Krak NC, Lammertsma AA, Boellaard R. Partial volume correction strategies for quantitative FDG PET in oncology. Eur J Nucl Med Mol Imaging 2010;37:1679–87.
Goethals I, D’Asseler Y, Dobbeleir A, Deblaere K, Ham H. The effect of acquisition time on visual and semi-quantitative analysis of F-18 FDG-PET studies in patients with head and neck cancer. Nucl Med Commun 2010;31:227–31.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47.
Sattler B, Lee J, Lonsdale M, Coche E. PET/CT (and CT) instrumentation, image reconstruction and data transfer for radiotherapy planning. Radiother Oncol 2010;96:288–97.
Zaidi H. Comparative evaluation of scatter correction techniques in 3D positron emission tomography. Eur J Nucl Med 2000;27:1813–26.
Lammering G, De Ruysscher D, van Baardwijk A, Baumert BG, Borger J, Lutgens L, et al. The use of FDG-PET to target tumors by radiotherapy. Strahlenther Onkol 2010;186:471–81.
Bussink J, Kaanders J, van der Graaf W, Oyen W. PET-CT for radiotherapy treatment planning and response monitoring in solid tumors. Nat Rev Clin Oncol 2011;8:233–42.
Doweck I, Denys D, Robbins KT. Tumor volume predicts outcome for advanced head and neck cancer treated with targeted chemoradiotherapy. Laryngoscope 2002;112:1742–9.
Knegjens J, Hauptmann M, Pameijer F, Balm AJ, Hoebers FJ, de Bois JA, et al. Tumor volume as prognostic factor in chemoradiation for advanced head and neck cancer. Head Neck 2011;33:375–82.
Plataniotis G, Theofanopoulou M, Kalogera-Fountzila A, Haritanti A, Ciuleanou E, Ghilezan N, et al. Prognostic impact of tumor volumetry in patients with locally advanced head-and-neck carcinoma (non-nasopharyngeal) treated by radiotherapy alone or combined radiochemotherapy in a randomized trial. Int J Radiat Oncol Biol Phys 2004;59:1018–26.
Yan H, Wang R, Zhao F, Zhu K, Jiang S, Zhao W, et al. Measurement of tumor volume by PET to evaluate prognosis in patients with advanced non-small cell lung cancer treated by non-surgical therapy. Acta Radiol 2011;52:646–50.
Gulec S, Suthar R, Barot T, Pennington K. The prognostic value of functional tumor volume and total lesion glycolysis in patients with colorectal cancer liver metastases undergoing (90)Y selective internal radiation therapy plus chemotherapy. Eur J Nucl Med Mol Imaging 2011;38:1289–95.
Chung H, Kim J, Han K, Eo JS, Kang KW, Park NH, et al. Prognostic value of metabolic tumor volume measured by FDG-PET/CT in patients with cervical cancer. Gynecol Oncol 2011;120:270–4.
Schinagl D, Span P, Oyen W, Kaanders J. Can FDG PET predict radiation treatment outcome in head and neck cancer? Results of a prospective study. Eur J Nucl Med Mol Imaging 2011;38:1449–58.
Hatt M, Visvikis D, Pradier O, Cheze-le-Rest C. Baseline 18F-FDG PET image-derived parameters for therapy response prediction in oesophageal cancer. Eur J Nucl Med Mol Imaging 2011;38:1595–606.
Cazaentre T, Morschhauser F, Vermandel M, Betrouni N, Prangère T, Steinling M, Huglo D. Pre-therapy 18F-FDG PET quantitative parameters help in predicting the response to radioimmunotherapy in non-Hodgkin lymphoma. Eur J Nucl Med Mol Imaging 2010;37:494–504.
Hatt M, Cheze Le Rest C, Albaraghach N, Pradier O, Visvikis D. PET functional volume delineation: a robustness and repeatability study. Eur J Nucl Med Mol Imaging 2011;38:663–72.
Hatt M, Cheze-Le Rest C, Aboagye E, Kenny LM, Rosso L, Turkheimer FE, et al. Reproducibility of 18F-FDG and 3′-deoxy-3′-18F-fluorothymidine PET tumor volume measurements. J Nucl Med 2010;51:1368–76.
Boellaard R, Krak N, Hoekstra O, Lammertsma A. Effects of noise, image resolution, and ROI definition on the accuracy of standard uptake values: a simulation study. J Nucl Med 2004;45:1519–27.
Wahl R, Jacence H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med 2009;50:122S–50S.
Juweid ME, Cheson BD. Positron-emission tomography and assessment of cancer therapy. N Engl J Med 2006;354:496–507.
Weber WA, Wieder H. Monitoring chemotherapy and radiotherapy of solid tumors. Eur J Nucl Med Mol Imaging 2006;33:27–37.
Acknowledgments
No financial support was received. The hours of practical and administrative work by Dr. Berwouts Dieter and Mw. Blanken Tamara as well as the support of the whole administrative, nursing, radiopharmaceutical, engineering and medical staff of the nuclear medicine department at the University Hospital of Ghent were all much appreciated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mertens, J., Dobbeleir, A., Ham, H. et al. Standardized added metabolic activity (SAM): a partial volume independent marker of total lesion glycolysis in liver metastases. Eur J Nucl Med Mol Imaging 39, 1441–1448 (2012). https://doi.org/10.1007/s00259-012-2166-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-012-2166-0