Abstract
Purpose
We investigated influences of pretargeting variables, tumor location, and radionuclides in pretargeted radioimmunotherapy (PRIT) as well as estimated tumor absorbed doses.
Methods
LS-174T human colonic carcinoma cells expressing carcinoembryonic antigen (CEA) were inoculated in nude mice. Biodistribution of a bispecific anti-CEA x anti-hapten antibody, TF2, and of a TF2-pretargeted peptide was assessed and a multi-compartment pharmacokinetic model was devised. Tissue absorbed doses were calculated for 131I, 177Lu, 90Y, 211At, and 213Bi using realistic specific activities.
Results
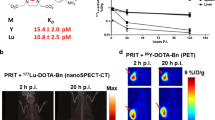
Under conditions optimized for tumor imaging (10:1 TF2 to peptide molar ratio, interval time 15–24 h), tumor uptake reached ∼9 ID/g in subcutaneous tumors at 2 h with very low accretion in normal tissues (tumor to blood ratio >20:1 after 2 h). For a low dose of peptide (0.04 nmol), 211At is predicted to deliver a high absorbed dose to tumors [41.5 Gy considering a relative biologic effect (RBE) of 5], kidneys being dose-limiting. 90Y and 213Bi would also deliver high absorbed doses to tumor (18.6 for 90Y and 26.5 Gy for 213Bi, taking RBE into account, for 0.1 nmol) and acceptable absorbed doses to kidneys. With hepatic metastases, a twofold higher tumor absorbed dose is expected. Owing to the low activities measured in blood, the bone marrow absorbed dose is expected to be without significant toxicity.
Conclusion
Pretargeting achieves high tumor uptake and higher tumor to background ratios compared to direct RIT. Short-lived radionuclides are predicted to deliver high tumor absorbed doses especially 211At, with kidneys being the dose-limiting organ. 177Lu and 131I should be considered for repeated injections.





Similar content being viewed by others
References
Simmonds PC. Palliative chemotherapy for advanced colorectal cancer: systematic review and meta-analysis. Colorectal Cancer Collaborative Group. BMJ 2000;321:531–5.
Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335–42.
Zhu H, Baxter LT, Jain RK. Potential and limitations of radioimmunodetection and radioimmunotherapy with monoclonal antibodies. J Nucl Med 1997;38:731–41.
Tempero M, Leichner P, Baranowska-Kortylewicz J, Harrison K, Augustine S, Schlom J, et al. High-dose therapy with 90yttrium-labeled monoclonal antibody CC49: a phase I trial. Clin Cancer Res 2000;6(8):3095–102.
Goldenberg DM, Chatal JF, Barbet J, Boerman O, Sharkey RM. Cancer imaging and therapy with bispecific antibody pretargeting. Update Cancer Ther 2007;2(1):19–31.
Le Doussal JM, Martin M, Gautherot E, Delaage M, Barbet J. In vitro and in vivo targeting of radiolabeled monovalent and divalent haptens with dual specificity monoclonal antibody conjugates: enhanced divalent hapten affinity for cell-bound antibody conjugate. J Nucl Med 1989;30:1358–66.
Le Doussal JM, Gruaz-Guyon A, Martin M, Gautherot E, Delaage M, Barbet J. Targeting of indium-111-labeled bivalent hapten to human melanoma mediated by bispecific monoclonal antibody conjugates: imaging of tumors hosted in nude mice. Cancer Res 1990;50:3445–52.
Gautherot E, Le Doussal JM, Bouhou J, Manetti C, Martin M, Rouvier E, et al. Delivery of therapeutic doses of radioiodine using bispecific antibody-targeted bivalent haptens. J Nucl Med 1998;39(11):1937–43.
Sharkey RM, Karacay H, Vallabhajosula S, McBride WJ, Rossi EA, Chang CH, et al. Metastatic human colonic carcinoma: molecular imaging with pretargeted SPECT and PET in a mouse model. Radiology 2008;246:497–507.
Rossi EA, Goldenberg DM, Cardillo TM, McBride WJ, Sharkey RM, Chang CH. Stably tethered multifunctional structures of defined composition made by the dock and lock method for use in cancer targeting. Proc Natl Acad Sci U S A 2006;103(18):6841–6.
Goldenberg DM, Rossi EA, Sharkey RM, McBride WJ, Chang CH. Multifunctional antibodies by the dock-and-lock method for improved cancer imaging and therapy by pretargeting. J Nucl Med 2008;49:158–63.
Bitar A, Lisbona A, Thedrez P, Sai Maurel C, Le Forestier D, Barbet J, et al. A voxel-based mouse for internal dose calculations using Monte Carlo simulations (MNCP). Phys Med Biol 2007;52:1013–25.
McBride WJ, Zanzonico P, Sharkey RM, Norén C, Karacay H, Rossi EA, et al. Bispecific antibody pretargeting PET (immunoPET) with an 124I-labeled hapten-peptide. J Nucl Med 2006;47:1678–88.
Frampas E, Saï-Maurel C, Thedrez P, Remaud-Le Saëc P, Faivre-Chauvet A, Barbet J. The intraportal injection model for liver metastasis: advantages of associated bioluminescence to assess tumor growth and influences on tumor uptake of radiolabeled anti-carcinoembryonic antigen antibody. Nucl Med Commun 2011;32(2):147–53.
Schoffelen R, van der Graaf WTA, Franssen G, Sharkey RM, Goldenberg DM, McBride WJ, et al. Pretargeted 177Lu radioimmunotherapy of carcinoembryonic antigen-expressing human colonic tumors in mice. J Nucl Med 2010;51:1780–7.
Karacay H, Sharkey RM, Gold DV, Ragland DR, McBride WJ, Rossi EA, et al. Pretargeted radioimunotherapy of pancreatic cancer xenografts: TF10-90Y-IMP-288 alone and combined with gemcitabine. J Nucl Med 2009;50:2008–16.
Bitar A, Lisbona A, Bardiès M. S-factor calculations for mouse models using Monte-Carlo simulations. Q J Nucl Med Mol Imaging 2007;51:343–51.
Bardiès M, Chatal JF. Absorbed doses for internal radiotherapy from 22 beta-emitting radionuclides: beta dosimetry of small spheres. Phys Med Biol 1994;39(6):961–81.
Stabin MG, Sparks RB, Crowe E. OLINDA/EXM: the second-generation personal computer software for internal dose assessment in nuclear medicine. J Nucl Med 2005;46(6):1023–7.
MIRD radionuclide data and decay schemes, 2nd ed. Eckerman KF, Endo A, editors. Reston: The Society of Nuclear Medicine; 2008.
Sgouros G, Roeske JC, McDevitt MR, Palm S, Allen BJ, Fisher DR, et al. MIRD Pamphlet No. 22 (abridged): radiobiology and dosimetry of α-particle emitters for targeted radionuclide therapy. J Nucl Med 2010;51:311–28.
Elgqvist J, Bernhardt P, Hultborn R, Jensen H, Karlsson B, Lindegren S, et al. Myelotoxicity and RBE of 211At-conjugated monoclonal antibodies compared with 99mTc-conjugated monoclonal antibodies and 60Co irradiation in nude mice. J Nucl Med 2005;46:464–71.
Bäck T, Haraldsson B, Hultborn R, Jensen H, Johansson ME, Lindegren S, et al. Glomerular filtration rate after alpha-radioimmunotherapy with 211At-MX35-F(ab′)2: a long-term study of renal function in nude mise. Cancer Biother Radiopharm 2009;24(6):649–58.
Sharkey RM, McBride WJ, Karacay H, Chang K, Griffiths GL, Hansen HJ, et al. A universal pretargeting system for cancer detection and therapy using bispecific antibody. Cancer Res 2003;63:354–63.
Karacay H, Sharkey RM, McBride WJ, Griffiths GL, Qu Z, Chang K, et al. Pretargeting for cancer radioimmunotherapy with bispecific antibodies: role of the bispecific antibody’s valency for the tumor target antigen. Bioconjug Chem 2002;13(5):1054–70.
Sharkey RM, Karacay H, Litwin S, Rossi EA, McBride WJ, Chang CH, et al. Improved therapeutic results by pretargeted radioimmunotherapy of non-Hodgkin’s lymphoma with a new recombinant, trivalent, anti-CD20, bispecific antibody. Cancer Res 2008;68(13):5282–90.
Sharkey RM, Rossi EA, McBride WJ, Chang CH, Goldenberg DM. Recombinant bispecific monoclonal antibodies prepared by the dock-and-lock strategy for pretargeted radioimmunotherapy. Semin Nucl Med 2010;40:190–203.
Cardillo TM, Karacay H, Goldenberg DM, Yeldell D, Chang CH, Modrak DE, et al. Improved targeting of pancreatic cancer: experimental studies of a new bispecific antibody, pretargeting enhancement system for immunoscintigraphy. Clin Cancer Res 2004;10:3552–61.
Sharkey RM, Karacay H, Cardillo TM, Chang CH, McBride WJ, Rossi EA, et al. Improving the delivery of radionuclides for imaging and therapy of cancer using pretargeting methods. Clin Cancer Res 2005;11(19 Suppl):7109s–21s.
Breeman WAP, de Jong M, Visser TJ, Erion JL, Krenning EP. Optimising conditions for radiolabelling of DOTA-peptides with 90Y, 111In and 177Lu at high specific activities. Eur J Nucl Med Mol Imaging 2003;30:917–20.
Brechbiel MW. Targeted α-therapy: past, present, future? Dalton Trans 2007;43:4918–28.
Chérel M, Davodeau F, Kraeber-Bodéré F, Chatal JF. Current status and perspectives in alpha radioimmunotherapy. Q J Nucl Med Mol Imaging 2006;50:322–9.
Sgouros G. Bone marrow dosimetry for radioimmunotherapy: theoretical considerations. J Nucl Med 1993;34(4):689–94.
Behr TM, Sharkey RM, Juweid ME, Dunn RM, Vagg RC, Ying Z, et al. Phase I/II clinical radioimmunotherapy with an iodine-131-labeled anti-carcinoembryonic antigen murine monoclonal antibody IgG. J Nucl Med 1997;38:858–70.
Thames HD, Hendry JH. Radiation-induced injury to tissues. In: Thames HD, editor. Fractionation in radiotherapy. London: Taylor and Francis; 1987. p. 1–21.
Behr TM, Béhé M, Stabin MG, Wehrmann E, Apostolidis C, Molinet R, et al. High-linear energy transfer (LET) α versus low-LET β emitters in radioimmunotherapy of solid tumors: therapeutic efficacy and dose-limiting toxicity of 213Bi- versus 90Y-labeled CO17-1A Fab′ fragments in a human colonic cancer model. Cancer Res 1999;59:2635–43.
Behr TM, Sgouros G, Stabin MG, Béhé M, Angerstein C, Blumenthal RD, et al. Studies on the red marrow dosimetry in radioimmunotherapy: an experimental investigation of factors influencing the radiation-induced myelotoxicity in therapy with β-, Auger/conversion electron-, or α-emitters. Clin Cancer Res 1999;5:3031s–43s.
Karagiannis TC. Comparison of different classes of radionuclides for potential use in radioimmunotherapy. Hell J Nucl Med 2007;10(2):82–8.
Sharkey RM, Primus FJ, Goldenberg DM. Antibody protein dose and radioimmunodetection of GW-39 human colon tumor xenografts. Int J Cancer 1987;39:611–7.
Vogel CA, Galmiche MC, Westermann P, Sun LQ, Pèlegrin A, Folli S, et al. Carcinoembryonic antigen expression, antibody localisation and immunophotodetection of human colon cancer liver metastases in nude mice: a model for radioimmunotherapy. Int J Cancer 1996;67:294–302.
Fidarova EF, El-Emir E, Boxer GM, Qureshi U, Dearling JLJ, Robson MP, et al. Microdistribution of targeted, fluorescently labeled anti-carcinoembryonic antigen antibody in metastatic colorectal cancer: implications for radioimmunotherapy. Clin Cancer Res 2008;14(9):2639–46.
Acknowledgments
We thank Robert M. Sharkey, Ph.D., for critical review of this manuscript. We are grateful to William McBride, Ph.D., for providing the peptides, and Chien-Hsing Chang, Ph.D., and Edmund A. Rossi, Ph.D., for the TF2 construct.
Financial support
This work was carried out with the financial support of the European Commission FP7 Collaborative Project program, contract TARCC n HEALTH-F2-2007-201962.
Conflicts of interest
D.M. Goldenberg is employed by or has financial interest in Immunomedics, Inc., and/or IBC Pharmaceuticals, Inc. The other authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Frampas, E., Maurel, C., Remaud-Le Saëc, P. et al. Pretargeted radioimmunotherapy of colorectal cancer metastases: models and pharmacokinetics predict influence of the physical and radiochemical properties of the radionuclide. Eur J Nucl Med Mol Imaging 38, 2153–2164 (2011). https://doi.org/10.1007/s00259-011-1903-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-011-1903-0