Abstract
Purpose
Several lines of evidence imply early alterations in metabolic, dopaminergic and endocannabinoid neurotransmission in Huntington’s disease (HD). Using [18F]MK-9470 and small animal PET, we investigated cerebral changes in type 1 cannabinoid (CB1) receptor binding in the quinolinic acid (QA) rat model of HD in relation to glucose metabolism, dopamine D2 receptor availability and amphetamine-induced turning behaviour.
Methods
Twenty-one Wistar rats (11 QA and 10 shams) were investigated. Small animal PET acquisitions were conducted on a Focus 220 with approximately 18 MBq of [18F]MK-9470, [18F]FDG and [11C]raclopride. Relative glucose metabolism and parametric CB1 receptor and D2 binding images were anatomically standardized to Paxinos space and analysed voxel-wise using Statistical Parametric Mapping (SPM2).
Results
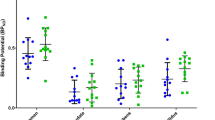
In the QA model, [18F]MK-9470 uptake, glucose metabolism and D2 receptor binding were reduced in the ipsilateral caudate-putamen by 7, 35 and 77%, respectively (all p < 2.10−5), while an increase for these markers was observed on the contralateral side (>5%, all p < 7.10−4). [18F]MK-9470 binding was also increased in the cerebellum (p = 2.10−5), where it was inversely correlated to the number of ipsiversive turnings (p = 7.10−6), suggesting that CB1 receptor upregulation in the cerebellum is related to a better functional outcome. Additionally, glucose metabolism was relatively increased in the contralateral hippocampus, thalamus and sensorimotor cortex (p = 1.10−6).
Conclusion
These data point to in vivo changes in endocannabinoid transmission, specifically for CB1 receptors in the QA model, with involvement of the caudate-putamen, but also distant regions of the motor circuitry, including the cerebellum. These data also indicate the occurrence of functional plasticity on metabolism, D2 and CB1 neurotransmission in the contralateral hemisphere.



Similar content being viewed by others
References
The Huntington’s Disease Collaborative Research Group. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. The Huntington’s Disease Collaborative Research Group. Cell 1993;72:971–83.
Vonsattel JP, Myers RH, Stevens TJ, Ferrante RJ, Bird ED, Richardson Jr EP. Neuropathological classification of Huntington’s disease. J Neuropathol Exp Neurol 1985;44:559–77.
Fernández-Ruiz J. The endocannabinoid system as a target for the treatment of motor dysfunction. Br J Pharmacol 2009;156:1029–40.
Glass M, Dragunow M, Faull RL. The pattern of neurodegeneration in Huntington’s disease: a comparative study of cannabinoid, dopamine, adenosine and GABA(A) receptor alterations in the human basal ganglia in Huntington’s disease. Neuroscience 2000;97:505–19.
Lastres-Becker I, Berrendero F, Lucas JJ, Martín-Aparicio E, Yamamoto A, Ramos JA, et al. Loss of mRNA levels, binding and activation of GTP-binding proteins for cannabinoid CB1 receptors in the basal ganglia of a transgenic model of Huntington’s disease. Brain Res 2002;929:236–42.
Naver B, Stub C, Møller M, Fenger K, Hansen AK, Hasholt L, et al. Molecular and behavioral analysis of the R6/1 Huntington’s disease transgenic mouse. Neuroscience 2003;122:1049–57.
McCaw EA, Hu H, Gomez GT, Hebb AL, Kelly ME, Denovan-Wright EM. Structure, expression and regulation of the cannabinoid receptor gene (CB1) in Huntington’s disease transgenic mice. Eur J Biochem 2004;271:4909–20.
Denovan-Wright EM, Robertson HA. Cannabinoid receptor messenger RNA levels decrease in a subset of neurons of the lateral striatum, cortex and hippocampus of transgenic Huntington’s disease mice. Neuroscience 2000;98:705–13.
Dowie MJ, Bradshaw HB, Howard ML, Nicholson LF, Faull RL, Hannan AJ, et al. Altered CB1 receptor and endocannabinoid levels precede motor symptom onset in a transgenic mouse model of Huntington’s disease. Neuroscience 2009;163:456–65.
Lastres-Becker I, Hansen HH, Berrendero F, De Miguel R, Pérez-Rosado A, Manzanares J, et al. Alleviation of motor hyperactivity and neurochemical deficits by endocannabinoid uptake inhibition in a rat model of Huntington’s disease. Synapse 2002;44:23–35.
Glass M, van Dellen A, Blakemore C, Hannan AJ, Faull RL. Delayed onset of Huntington’s disease in mice in an enriched environment correlates with delayed loss of cannabinoid CB1 receptors. Neuroscience 2004;123:207–12.
Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, et al. Cannabinoid receptor localization in brain. Proc Natl Acad Sci U S A 1990;87:1932–6.
Katona I, Freund TF. Endocannabinoid signaling as a synaptic circuit breaker in neurological disease. Nat Med 2008;14:923–30.
Burns HD, Van Laere K, Sanabria-Bohórquez S, Hamill TG, Bormans G, Eng WS, et al. [18F]MK-9470, a positron emission tomography (PET) tracer for in vivo human PET brain imaging of the cannabinoid-1 receptor. Proc Natl Acad Sci U S A 2007;104:9800–5.
Casteels C, Lauwers E, Baitar A, Bormans G, Baekelandt V, Van Laere K. In vivo type 1 cannabinoid receptor mapping in the 6-hydroxydopamine lesion rat model of Parkinson’s disease. Brain Res 2010;1316:153–62.
Beal MF, Kowall NW, Ellison DW, Mazurek MF, Swartz KJ, Martin JB. Replication of the neurochemical characteristics of Huntington’s disease by quinolinic acid. Nature 1986;321:168–71.
Beal MF, Kowall NW, Swartz KJ, Ferrante RJ, Martin JB. Differential sparing of somatostatin-neuropeptide Y and cholinergic neurons following striatal excitotoxin lesions. Synapse 1989;3:38–47.
Antonini A, Leenders KL, Spiegel R, Meier D, Vontobel P, Weigell-Weber M, et al. Striatal glucose metabolism and dopamine D2 receptor binding in asymptomatic gene carriers and patients with Huntington’s disease. Brain 1996;119(Pt 6):2085–95.
Araujo DM, Cherry SR, Tatsukawa KJ, Toyokuni T, Kornblum HI. Deficits in striatal dopamine D(2) receptors and energy metabolism detected by in vivo microPET imaging in a rat model of Huntington’s disease. Exp Neurol 2000;166:287–97.
Vivó M, Camón L, de Vera N, Martínez E. Lesion of substantia nigra pars compacta by the GluR5 agonist ATPA. Brain Res 2002;955:104–14.
Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego: Academic; 1998.
Camón L, de Vera N, Martínez E. Polyamine metabolism and glutamate receptor agonists-mediated excitotoxicity in the rat brain. J Neurosci Res 2001;66:1101–11.
Visnyei K, Tatsukawa KJ, Erickson RI, Simonian S, Oknaian N, Carmichael ST, et al. Neural progenitor implantation restores metabolic deficits in the brain following striatal quinolinic acid lesion. Exp Neurol 2006;197:465–74.
Casteels C, Vermaelen P, Nuyts J, Van Der Linden A, Baekelandt V, Mortelmans L, et al. Construction and evaluation of multitracer small-animal PET probabilistic atlases for voxel-based functional mapping of the rat brain. J Nucl Med 2006;47:1858–66.
Farde L, Ito H, Swahn CG, Pike VW, Halldin C. Quantitative analyses of carbonyl-carbon-11-WAY-100635 binding to central 5-hydroxytryptamine-1A receptors in man. J Nucl Med 1998;39:1965–71.
Casteels C, Bormans G, Van Laere K. The effect of anaesthesia on [(18)F]MK-9470 binding to the type 1 cannabinoid receptor in the rat brain. Eur J Nucl Med Mol Imaging 2010;37:1164–73.
Terry GE, Liow JS, Zoghbi SS, Hirvonen J, Farris AG, Lerner A, et al. Quantitation of cannabinoid CB1 receptors in healthy human brain using positron emission tomography and an inverse agonist radioligand. Neuroimage 2009;48:362–70.
Ichise M, Liow JS, Lu JQ, Takano A, Model K, Toyama H, et al. Linearized reference tissue parametric imaging methods: application to [11C]DASB positron emission tomography studies of the serotonin transporter in human brain. J Cereb Blood Flow Metab 2003;23:1096–112.
Goffin K, Bormans G, Casteels C, Bosier B, Lambert DM, Grachev ID, et al. An in vivo [(18)F]MK-9470 microPET study of type 1 cannabinoid receptor binding in Wistar rats after chronic administration of valproate and levetiracetam. Neuropharmacology 2008;54:1103–6.
van Kuyck K, Casteels C, Vermaelen P, Bormans G, Nuttin B, Van Laere K. Motor- and food-related metabolic cerebral changes in the activity-based rat model for anorexia nervosa: a voxel-based microPET study. Neuroimage 2007;35:214–21.
Casanova R, Srikanth R, Baer A, Laurienti PJ, Burdette JH, Hayasaka S, et al. Biological parametric mapping: a statistical toolbox for multimodality brain image analysis. Neuroimage 2007;34:137–43.
Maccarrone M, Battista N, Centonze D. The endocannabinoid pathway in Huntington’s disease: a comparison with other neurodegenerative diseases. Prog Neurobiol 2007;81:349–79.
Scherfler C, Scholz SW, Donnemiller E, Decristoforo C, Oberladstätter M, Stefanova N, et al. Evaluation of [123I]IBZM pinhole SPECT for the detection of striatal dopamine D2 receptor availability in rats. Neuroimage 2005;24:822–31.
Köfalvi A, Rodrigues RJ, Ledent C, Mackie K, Vizi ES, Cunha RA, et al. Involvement of cannabinoid receptors in the regulation of neurotransmitter release in the rodent striatum: a combined immunochemical and pharmacological analysis. J Neurosci 2005;25:2874–84.
Uchigashima M, Narushima M, Fukaya M, Katona I, Kano M, Watanabe M. Subcellular arrangement of molecules for 2-arachidonoyl-glycerol-mediated retrograde signaling and its physiological contribution to synaptic modulation in the striatum. J Neurosci 2007;27:3663–76.
Cepeda C, Wu N, André VM, Cummings DM, Levine MS. The corticostriatal pathway in Huntington’s disease. Prog Neurobiol 2007;81:253–71.
Ellison DW, Beal MF, Mazurek MF, Malloy JR, Bird ED, Martin JB. Amino acid neurotransmitter abnormalities in Huntington’s disease and the quinolinic acid animal model of Huntington’s disease. Brain 1987;110(Pt 6):1657–73.
Pintor A, Tebano MT, Martire A, Grieco R, Galluzzo M, Scattoni ML, et al. The cannabinoid receptor agonist WIN 55,212-2 attenuates the effects induced by quinolinic acid in the rat striatum. Neuropharmacology 2006;51:1004–12.
Walker FO. Huntington’s disease. Lancet 2007;369:218–28.
Fusco FR, Martorana A, Giampà C, De March Z, Farini D, D’Angelo V, et al. Immunolocalization of CB1 receptor in rat striatal neurons: a confocal microscopy study. Synapse 2004;53:159–67.
Ariano MA, Aronin N, Difiglia M, Tagle DA, Sibley DR, Leavitt BR, et al. Striatal neurochemical changes in transgenic models of Huntington’s disease. J Neurosci Res 2002;68:716–29.
Wang X, Sarkar A, Cicchetti F, Yu M, Zhu A, Jokivarsi K, et al. Cerebral PET imaging and histological evidence of transglutaminase inhibitor cystamine induced neuroprotection in transgenic R6/2 mouse model of Huntington’s disease. J Neurol Sci 2005;231:57–66.
Brownell AL, Chen YI, Yu M, Wang X, Dedeoglu A, Cicchetti F, et al. 3-Nitropropionic acid-induced neurotoxicity—assessed by ultra high resolution positron emission tomography with comparison to magnetic resonance spectroscopy. J Neurochem 2004;89:1206–14.
Ishiwata K, Ogi N, Hayakawa N, Oda K, Nagaoka T, Toyama H, et al. Adenosine A2A receptor imaging with [11C]KF18446 PET in the rat brain after quinolinic acid lesion: comparison with the dopamine receptor imaging. Ann Nucl Med 2002;16:467–75.
Kuwert T, Lange HW, Langen KJ, Herzog H, Aulich A, Feinendegen LE. Cortical and subcortical glucose consumption measured by PET in patients with Huntington’s disease. Brain 1990;113(Pt 5):1405–23.
Jueptner M, Weiller C. Review: does measurement of regional cerebral blood flow reflect synaptic activity? Implications for PET and fMRI. Neuroimage 1995;2:148–56.
Dihné M, Block F, Korr H, Töpper R. Time course of glial proliferation and glial apoptosis following excitotoxic CNS injury. Brain Res 2001;902:178–89.
Moresco RM, Lavazza T, Belloli S, Lecchi M, Pezzola A, Todde S, et al. Quinolinic acid induced neurodegeneration in the striatum: a combined in vivo and in vitro analysis of receptor changes and microglia activation. Eur J Nucl Med Mol Imaging 2008;35:704–15.
Nikolaus S, Larisch R, Beu M, Forutan F, Vosberg H, Müller-Gärtner HW. Bilateral increase in striatal dopamine D2 receptor density in the 6-hydroxydopamine-lesioned rat: a serial in vivo investigation with small animal PET. Eur J Nucl Med Mol Imaging 2003;30:390–5.
Ferré S, Fuxe K. Dopamine denervation leads to an increase in the intramembrane interaction between adenosine A2 and dopamine D2 receptors in the neostriatum. Brain Res 1992;594:124–30.
Alloway KD, Smith JB, Beauchemin KJ, Olson ML. Bilateral projections from rat MI whisker cortex to the neostriatum, thalamus, and claustrum: forebrain circuits for modulating whisking behavior. J Comp Neurol 2009;515:548–64.
Pritzel M, Huston JP, Sarter M. Behavioral and neuronal reorganization after unilateral substantia nigra lesions: evidence for increased interhemispheric nigrostriatal projections. Neuroscience 1983;9:879–88.
Asúa T, Bilbao A, Gorriti MA, Lopez-Moreno JA, Del Mar Alvarez M, Navarro M, et al. Implication of the endocannabinoid system in the locomotor hyperactivity associated with congenital hypothyroidism. Endocrinology 2008;149:2657–66.
Scattoni ML, Valanzano A, Popoli P, Pezzola A, Reggio R, Calamandrei G. Progressive behavioural changes in the spatial open-field in the quinolinic acid rat model of Huntington’s disease. Behav Brain Res 2004;152:375–83.
Song J, Lee ST, Kang W, Park JE, Chu K, Lee SE, et al. Human embryonic stem cell-derived neural precursor transplants attenuate apomorphine-induced rotational behavior in rats with unilateral quinolinic acid lesions. Neurosci Lett 2007;423:58–61.
Vazey EM, Chen K, Hughes SM, Connor B. Transplanted adult neural progenitor cells survive, differentiate and reduce motor function impairment in a rodent model of Huntington’s disease. Exp Neurol 2006;199:384–96.
Barrero FJ, Ampuero I, Morales B, Vives F, de Dios Luna Del Castillo J, Hoenicka J, et al. Depression in Parkinson’s disease is related to a genetic polymorphism of the cannabinoid receptor gene (CNR1). Pharmacogenomics J 2005;5:135–41.
Van Laere KJ, Versijpt J, Koole M, Vandenberghe S, Lahorte P, Lemahieu I, et al. Experimental performance assessment of SPM for SPECT neuroactivation studies using a subresolution sandwich phantom design. Neuroimage 2002;16:200–16.
Brasted PJ, Humby T, Dunnett SB, Robbins TW. Unilateral lesions of the dorsal striatum in rats disrupt responding in egocentric space. J Neurosci 1997;17:8919–26.
Kung MP, Kung HF. Mass effect of injected dose in small rodent imaging by SPECT and PET. Nucl Med Biol 2005;32:673–8.
Acknowledgements
Merck & Co., Inc. is acknowledged for the availability of the [18F]MK-9470 precursor, and for their critical revision of this manuscript and their improving suggestions. The authors thank Peter Vermaelen for his assistance in data acquisition, as well as the Leuven PET radiopharmacy team for tracer preparations. Financial support of the Research Council of the Katholieke Universiteit Leuven (OT/05/58), the Fund for Scientific Research, Flanders, Belgium (FWO/G.0548.06), the K.U. Leuven Molecular Small Animal Imaging Center (KUL EF/05/08), and the Institute for the Promotion of Innovation by Science and Technology in Flanders (SBO50151) is gratefully acknowledged. Part of this work is also performed under the European Commission FP6 project Diagnostic Molecular Imaging (DIMI), LSHB-CT-2005-512146.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Casteels, C., Martinez, E., Bormans, G. et al. Type 1 cannabinoid receptor mapping with [18F]MK-9470 PET in the rat brain after quinolinic acid lesion: a comparison to dopamine receptors and glucose metabolism. Eur J Nucl Med Mol Imaging 37, 2354–2363 (2010). https://doi.org/10.1007/s00259-010-1574-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-010-1574-2