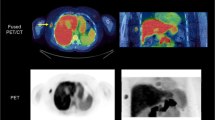
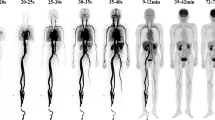
Abstract
Purpose
[11C]Acetate (C-AC) is a general PET tracer of cellular carbon flux and useful for clinical imaging in heart disease as well as prostate cancer and other tumours. C-AC has a high (70%) whole-body extraction fraction, proportional to blood flow in many organs. Trapping is related to organ-specific enzymatic activation and formation of [11C]-acetyl-CoA, the fate of which has been well characterized. Due to the logistic challenges with C-AC, 2-[18F]fluoroacetate (F-AC) has been proposed as a marker for prostate cancer imaging.
Method
We evaluated the potential of F-AC as a tracer for imaging blood flow and early enzymatic steps in the intermediary metabolism. C-AC and F-AC were injected serially in three cynomolgus monkeys and one domestic pig and scanned using PET/CT. A dynamic scan covering heart and liver was followed by repeated whole-body imaging. Kinetic patterns were compared for the myocardium, liver, blood and other organs.
Results
C-AC kinetics and organ distribution in both species were similar to those previously established in man. In contrast, F-AC showed prolonged blood retention, no detectable trapping in myocardium or salivary glands, rapid clearance from liver and extensive excretion to bile and urine. Massive defluorination was seen in the pig, resulting in intense skeletal activity.
Conclusion
2-[18F]Fluoroacetate cannot be regarded as a functional analogue of 1-[11C]acetate in normal physiology and appears to be of little use for studies of organ blood flow, intermediary metabolism or lipid synthesis.




Similar content being viewed by others
References
Hara T, Kondo T, Hara T, Kosaka N. Use of 18F-choline and 11C-choline as contrast agents in positron emission tomography imaging-guided stereotactic biopsy sampling of gliomas. J Neurosurg 2003;99(3):474–9
Ishiwata K, Kawamura K, Wang WF, Furumoto S, Kubota K, Pascali C, et al. Evaluation of O-[11C]methyl-L-tyrosine and O-[18F]fluoromethyl-L-tyrosine as tumor imaging tracers by PET. Nucl Med Biol 2004;31(2):191–8. doi:10.1016/j.nucmedbio.2003.07.004
Krohn KA, Mankoff DA, Eary JF. Imaging cellular proliferation as a measure of response to therapy. J Clin Pharmacol 2001;Suppl:96S–103S.
Vander Borght T, Pauwels S, Lambotte L, Labar D, De Maeght S, Stroobandt G, et al. Brain tumor imaging with PET and 2-[carbon-11]thymidine. J Nucl Med 1994;35(6):974–82
Krebs HA The tricarboxylic acid cycle. Harvey Lect 1948;Series 44:165–99
Yoshimoto M, Waki A, Yonekura Y, Sadato N, Murata T, Omata N, et al. Characterization of acetate metabolism in tumor cells in relation to cell proliferation: acetate metabolism in tumor cells. Nucl Med Biol 2001;28(2):117–22. doi:10.1016/S0969-8051(00)00195-5
Mittendorfer B, Sidossis LS, Walser E, Chinkes DL, Wolfe RR. Regional acetate kinetics and oxidation in human volunteers. Am J Physiol 1998;274(6 Pt 1):E978–83
Sörensen J, Andrén B, Blomquist G, Ståhle E, Långström B, Heidenstierna G. The central circulation in congestive heart failure non-invasively evaluated with dynamic positron emission tomography. Clin Physiol Funct Imaging 2006;26(3):171–7. doi:10.1111/j.1475-097X.2006.00670.x
Chen S, Ho C, Feng D, Chi Z. Tracer kinetic modeling of 11C-acetate applied in the liver with positron emission tomography. IEEE Trans Med Imaging 2004;23(4):426–32. doi:10.1109/TMI.2004.824229
Luong A, Hannah VC, Brown MS, Goldstein JL. Molecular characterization of human acetyl-CoA synthetase, an enzyme regulated by sterol regulatory element-binding proteins. J Biol Chem 2000;275(34):26458–66. doi:10.1074/jbc.M004160200
Fujino T, Kondo J, Ishikawa M, Morikawa K, Yamamoto TT. Acetyl-CoA synthetase 2, a mitochondrial matrix enzyme involved in the oxidation of acetate. J Biol Chem 2001;276(14):11420–6. doi:10.1074/jbc.M008782200
Rigo P, de Landsheere C, Melon P, Kulbertus H. Imaging of myocardial metabolism by positron emission tomography. Cardiovasc Drugs Ther 1990;4(Suppl 4):847–51. doi:10.1007/BF00051291
Sun A, Sörensen J, Karlsson M, Turesson I, Langström B, Nilsson P, et al. 1-[11C]-acetate PET imaging in head and neck cancer—a comparison with 18F-FDG-PET: implications for staging and radiotherapy planning. Eur J Nucl Med Mol Imaging 2007;34(5):651–7. doi:10.1007/s00259-006-0298-9
Soloviev D, Fini A, Chierichetti F, Al-Nahhas A, Rubello D. PET imaging with 11C-acetate in prostate cancer: a biochemical, radiochemical and clinical perspective. Eur J Nucl Med Mol Imaging 2008;35(5):942–9. doi:10.1007/s00259-007-0662-4
Oyama N, Akino H, Kanamaru H, Suzuki Y, Muramoto S, Yonekura Y, et al. 11C-acetate PET imaging of prostate cancer. J Nucl Med 2002;43(2):181–6
Oyama N, Miller TR, Dehdashti F, Siegel BA, Fischer KC, Michalski JM, et al. 11C-acetate PET imaging of prostate cancer: detection of recurrent disease at PSA relapse. J Nucl Med 2003;44(4):549–55
Matthies A, Ezziddin S, Ulrich EM, Palmedo H, Biersack HJ, Bender H, et al. Imaging of prostate cancer metastases with 18F-fluoroacetate using PET/CT. Eur J Nucl Med Mol Imaging 2004;31(5):797. doi:10.1007/s00259-003-1437-1
Nanni C, Castellucci P, Farsad M, Rubello D, Fanti S. 11C/18F-choline PET or 11C/18F-acetate PET in prostate cancer: may a choice be recommended? Eur J Nucl Med Mol Imaging 2007;34(10):1704–5. doi:10.1007/s00259-007-0491-5
Ponde DE, Dence CS, Oyama N, Kim J, Tai YC, Laforest R, et al. 18F-fluoroacetate: a potential acetate analog for prostate tumor imaging—in vivo evaluation of 18F-fluoroacetate versus 11C-acetate. J Nucl Med 2007;48(3):420–8
Goncharov NV, Jenkins RO, Radilov AS. Toxicology of fluoroacetate: a review, with possible directions for therapy research. J Appl Toxicol 2006;26(2):148–61. doi:10.1002/jat.1118
Sykes TR, Ruth TJ, Adam MJ. Synthesis and murine tissue uptake of sodium [18F]fluoroacetate. Int J Rad Appl Instrum B 1986;13(5):497–500. doi:10.1016/0883-2897(86)90126-1
Kihlberg T, Valind S, Långström B. Synthesis of [1-11C], [2-11C], [1-11C](2H3) and [2-11C](2H3)acetate for in vivo studies of myocardium using PET. Nucl Med Biol 1994;21(8):1067–72. doi:10.1016/0969-8051(94)90178-3
Tang D, Li HJ, Chen J, Guo CW, Li P. Rapid and simple method for screening of natural antioxidants from Chinese herb Flos Lonicerae Japonicae by DPPH-HPLC-DAD-TOF/MS. J Sep Sci 2008;31(20):3519–26. doi:10.1002/jssc.200800173
Jeong JM, Lee DS, Chung JK, Lee MC, Koh CS, Kang SS. Synthesis of no-carrier-added [18F]fluoroacetate. J Labelled Comp Radiopharm 1997;39(5):395–9. doi:10.1002/(SICI)1099-1344(199705)39:5<395::AID-JLCR985>3.0.CO;2-4
van den Hoff J, Burchert W, Börner AR, Fricke H, Kühnel G, Meyer GJ, et al. [1-11C]Acetate as a quantitative perfusion tracer in myocardial PET. J Nucl Med 2001;42(8):1174–82
Dienel GA, Popp D, Drew PD, Ball K, Krisht A, Cruz NF. Preferential labeling of glial and meningial brain tumors with [2-(14)C]acetate. J Nucl Med 2001;42(8):1243–50
Hosoi R, Kashiwagi Y, Tokumura M, Abe K, Hatazawa J, Inoue O. Sensitive reduction in 14C-acetate uptake in a short-term ischemic rat brain. J Stroke Cerebrovasc Dis 2007;16(2):77–81. doi:10.1016/j.jstrokecerebrovasdis.2006.11.005
Acknowledgements
We thank My Quach, Annie Bjurebäck, and Maj Wiberg for expert technical assistance. The study was supported by Uppsala University Hospital, Sweden.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lindhe, Ö., Sun, A., Ulin, J. et al. [18F]Fluoroacetate is not a functional analogue of [11C]acetate in normal physiology. Eur J Nucl Med Mol Imaging 36, 1453–1459 (2009). https://doi.org/10.1007/s00259-009-1128-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-009-1128-7