Abstract
Purpose
The very early chemotherapeutic effects of the FOLFOX (fluorouracil, folinic acid, oxaliplatin) protocol were assessed in mice implanted with a human colorectal cell line. The aim of this study was to identify changes in gene expression patterns and to detect combinations of PET parameters that may be helpful in identifying treated tumours early after chemotherapy using dynamic PET studies.
Methods
A human colorectal cell line (HCT 116) was used in nude mice. Dynamic PET studies were performed in untreated (n = 13) and treated (n = 12) animals. The data were assessed using compartmental and noncompartmental analysis. The removed tumour specimens were assessed by gene array analysis to obtain quantitative information on gene expression.
Results
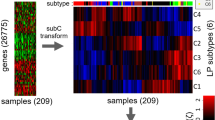
One chemotherapeutic treatment using the FOLFOX protocol resulted in an upregulation of 2,078 gene probes by more than 25%, while 2,254 probes were downregulated following treatment. The gene array data demonstrated primarily an enhancement of genes related to apoptosis. In particular, the apoptosis antigen 1 (APO-1), p21 and the G protein-coupled receptor 87 (G-87) were 2.6- to 3.3-fold upregulated as compared to the expression in untreated animals. There was a 100% separation of untreated and treated animals on the basis of these three genes. The SUV and the FDG kinetic parameters obtained by compartmental and noncompartmental fitting were not significantly different when individual parameters were compared between groups. However, classification analysis of the combination of the PET parameters VB, K1, k3, and influx revealed an overall accuracy of 84%. We were able to identify 91.7% (11/12) of the treated animals and 76.9% (10/13) of the untreated animals correctly using the classification analysis of PET data.
Conclusion
Even one chemotherapeutic treatment using FOLFOX has an impact on gene expression and significantly modulates FDG kinetics. Quantitative assessment of the tracer kinetics and the application of classification analysis to the data are promising tools to identify those tumours that demonstrate a chemotherapeutic effect very early following treatment.






Similar content being viewed by others
References
Bleiberg H, de Gramont A. Oxaliplatin plus 5-fluorouracil: clinical experience in patients with advanced colorectal cancer. Semin Oncol 1998;224:509–22.
Machover D, Diaz-Rubio E, de Gramont A, Schilf A, Gastiaburu JJ, Brienza S, et al. Two consecutive phase II studies of oxaliplatin (L-OHP) for treatment of patients with advanced colorectal carcinoma who were resistant to previous treatment with fluoropyrimidines. Ann Oncol 1996;7:95–8.
Louvet C, de Gramont A. Colorectal cancer: integrating oxaliplatin. Curr Treat Options Oncol 2003;4:405–11.
Grothey A, Goldberg RM. A review of oxaliplatin and its clinical use in colorectal cancer. Expert Opin Pharmacother 2004;5:2159–70.
Dimitrakopoulou-Strauss A, Strauss LG, Burger C, Ruehl A, Irngartinger G, Stremmel W, Rudi J. Prognostic aspects of F-18-FDG PET kinetics in patients with metastatic colorectal carcinoma receiving FOLFOX chemotherapy. J Nucl Med 2005;45:1480–7.
Strauss LG, Conti PS. The applications of PET in clinical oncology. J Nucl Med 1991;32:623–48.
Miyazawa H, Osmont A, Petit-Taboue MC, Tillet I, Travère JM, Young AR, et al. Determination of 18F-fluoro-2-deoxy-D-glucose rate constants in the anesthetized baboon brain with dynamic positron tomography. J Neurosci Methods 1993;50:263–72.
Sokoloff L, Smith CB. Basic principles underlying radioisotopic methods for assay of biochemical processes in vivo. In: Greitz T, Ingvar DH, Widén L, editors. The metabolism of the human brain studied with positron emission tomography. New York: Raven Press; 1983. p. 123–48.
Ohtake T, Kosaka N, Watanabe T, Yokoyama I, Moritan T, Masuo M, et al. Noninvasive method to obtain input function for measuring glucose utilization of thoracic and abdominal organs. J Nucl Med 1991;32:1432–8.
Pan L, Mikolajczyk K, Strauss LG, Haberkorn U, Dimitrakopoulou-Strauss A. Machine learning based parameter imaging and kinetic modelling of PET data. J Nucl Med 2007;48(Suppl 2):158P.
Dimitrakopoulou-Strauss A, Strauss LG, Mikolajczyk K, et al. On the fractal nature of dynamic positron emission tomography (PET) studies. World J Nucl Med 2003;2:306–13.
Teague TK, Hildeman D, Kedl RM, Mitchell T, Rees W, Schaefer BC, et al. Activation changes the spectrum but not the diversity of genes expressed by T cells. Proc Natl Acad Sci USA 1999;96:12691–6.
Strauss LG, Pan L, Koczan D, Klippel S, Mikolajczyk K, Burger C, et al. Fusion of positron emission tomography (PET) and gene array data: a new approach for the correlative analysis of molecular biological and clinical data. IEEE Trans Med Imaging 2007;26:804–12.
Chen PH, Lin CJ, Schölkopf B. A tutorial on v-support vector machines. Appl Stoch Models Bus Ind 2005;21:111–36.
Chu F, Wang L. Applications of support vector machines to cancer classification with microarray data. Int J Neural Syst 2005;15:475–84.
Guyon I, Weston J, Barnhill S, Vapnik V. Gene selection for cancer classification using support vector machines. Mach Learn 2002;46:389–422.
Fessler JA, Hero AO. Space-alternating generalized expectation-maximization algorithm. IEEE Trans Signal Process 1994;42:2664–77.
Green PJ. Bayesian reconstructions from emission tomography data using a modified EM algorithm. IEEE Trans Med Imaging 1990;9:84–93.
Alenius S, Ruotsalainen U. Bayesian image reconstruction for emission tomography based on median root prior. Eur J Nucl Med 1997;24:258–65.
Strauss LG, Koczan D, Klippel S, Pan L, Cheng C, Willis S, et al. Impact of angiogenesis-related gene expression on the tracer kinetics of 18F-FDG in colorectal tumours. J Nucl Med 2008;49:1238–44.
Gartel AL, Radhakrishnan SK. Lost in transcription: p21 repression, mechanisms, and consequences. Cancer Res 2005;65:3980–5.
Rakitina TV, Vasilevskaya IA, O’Dwyer PJ. Inhibition of G1/S transition potentiates oxaliplatin-induced cell death in colon cancer cell lines. Biochem Pharmacol 2007;73:1715–26.
Hayward RL, Macpherson JS, Cummings J, Monia BP, Smyth JF, Jodrell DI. Enhanced oxaliplatin-induced apoptosis following antisense Bcl-xl down-regulation is p53 and bax dependent: genetic evidence for specificity of the antisense effect. Mol Cancer Ther 2004;3:169–78.
Milovic-Holm K, Krieghoff E, Jensen K, Will H, Hofmann TG. FLASH links the CD95 signalling pathway to the cell nucleus and nuclear bodies. EMBO J 2007;26:391–401.
Iwase M, Watanabe H, Kondo G, Ohashi M, Nagumo M. Enhanced susceptibility of oral squamous cell carcinoma cell lines to FAS-mediated apoptosis by cisplatin and 5-fluorouracil. Int J Cancer 2003;106:619–25.
Elefsinioti AL, Bagos PG, Spyropoulos IC, Hamodrakas SJ. A database for G proteins and their interaction with GPCRs. BMC Bioinformatics 2004;5:208.
Glatt S, Halbauer D, Heindl S, Wernitznig A, Kozina D, Su KC, et al. hGPR87 contributes to viability of human cancer cells. Int J Cancer 2008;122:2008–16.
Kerley-Hamilton JS, Pike AM, Li N, DiRenzo J, Spinella MJ. A p53-dominant transcriptional response to cisplatin in testicular germ cell tumour-derived human embryonal carcinoma. Oncogene 2005;24:6090–100.
Strauss LG, Dimitrakopoulou-Strauss A, Koczan D, Bernd L, Haberkorn U, Ewerbeck V, et al. 18F-FDG kinetics and gene expression in giant cell tumours. J Nucl Med 2004;45:1528–35.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Strauss, L.G., Hoffend, J., Koczan, D. et al. Early effects of FOLFOX treatment of colorectal tumour in an animal model: assessment of changes in gene expression and FDG kinetics. Eur J Nucl Med Mol Imaging 36, 1226–1234 (2009). https://doi.org/10.1007/s00259-009-1102-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-009-1102-4