Abstract
Purpose
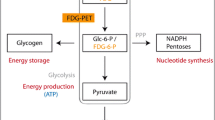
This study was designed to assess changes in glucose metabolism in rats administered single or repeated doses of MDMA.
Methods
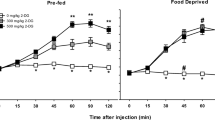
Two different experiments were performed: (1) A single-dose study with four groups receiving 20 mg/kg, 40 mg/kg, saline or heat, and (2) a repeated-dose study with two groups receiving three doses, at intervals of 2 h, of 5 mg/kg or saline. Rats were imaged using a dedicated small-animal PET scanner 1 h after single-dose administration or 7 days after repeated doses. Glucose metabolism was measured in 12 cerebral regions of interest. Rectal temperature and blood glucose were monitored.
Results
Peak body temperature was reached 1 h after MDMA administration. Blood glucose levels decreased significantly after MDMA administration. In the single-dose experiment, brain glucose metabolism showed hyperactivation in cerebellum and hypo-activation in the hippocampus, amygdala and auditory cortex. In the repeated-dose experiment, brain glucose metabolism did not show any significant change at day 7.
Conclusion
These results are the first to indicate that MDMA has the potential to produce significant hypoglycaemia. In addition, they show that MDMA alters glucose metabolism in components of the motor, limbic and somatosensory systems acutely but not on a long-term basis.







Similar content being viewed by others
References
Grob CS, Poland RE, Chang L, Ernst T. Psychobiologic effects of 3,4-methylenedioxymethamphetamine in humans: methodological considerations and preliminary observations. Behav Brain Res 1996;73(1–2):103–7.
Cole JC, Sumnall HR. Altered states: the clinical effects of ecstasy. Pharmacol Ther 2003;98(1):35–58.
Schmidt CJ, Taylor VL. Depression of rat brain tryptophan hydroxylase activity following the acute administration of methylenedioxymethamphetamine. Biochem Pharmacol 1987;36(23):4095–102.
Stone DM, Stahl DC, Hanson GR, Gibb JW. The effects of 3,4-methylenedioxymethamphetamine (MDMA) and 3,4-methylenedioxyamphetamine (MDA) on monoaminergic systems in the rat brain. Eur J Pharmacol 1986;128(1–2):41–8.
Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, et al. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse 2001;39(1):32–41.
Green AR, Cross AJ, Goodwin GM. Review of the pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA or “Ecstasy”). Psychopharmacology (Berl) 1995;119(3):247–60.
Obradovic T, Imel KM, White SR. Methylenedioxymethamphetamine-induced inhibition of neuronal firing in the nucleus accumbens is mediated by both serotonin and dopamine. Neuroscience 1996;74(2):469–81.
White SR, Obradovic T, Imel KM, Wheaton MJ. The effects of methylenedioxymethamphetamine (MDMA, “Ecstasy”) on monoaminergic neurotransmission in the central nervous system. Prog Neurobiol 1996;49(5):455–79.
Bolla KI, McCann UD, Ricaurte GA. Memory impairment in abstinent MDMA (“Ecstasy”) users. Neurology 1998;51(6):1532–7.
McCann UD, Szabo Z, Scheffel U, Dannals RF, Ricaurte GA. Positron emission tomographic evidence of toxic effect of MDMA (“Ecstasy”) on brain serotonin neurons in human beings. Lancet 1998;352(9138):1433–7.
Insel TR, Battaglia G, Johannessen JN, Marra S, De Souza EB. 3,4-Methylenedioxymethamphetamine (“ecstasy”) selectively destroys brain serotonin terminals in rhesus monkeys. J Pharmacol Exp Ther 1989;249(3):713–20.
Hatzidimitriou G, McCann UD, Ricaurte GA. Altered serotonin innervation patterns in the forebrain of monkeys treated with (+/−)3,4-methylenedioxymethamphetamine seven years previously: factors influencing abnormal recovery. J Neurosci 1999;19(12):5096–107.
Ricaurte GA, McCann UD, Szabo Z, Scheffel U. Toxicodynamics and long-term toxicity of the recreational drug, 3, 4-methylenedioxymethamphetamine (MDMA, ‘Ecstasy’). Toxicol Lett 2000;112–113:143–6.
Fantegrossi WE, Woolverton WL, Kilbourn M, Sherman P, Yuan J, Hatzidimitriou G, et al. Behavioral and neurochemical consequences of long-term intravenous self-administration of MDMA and its enantiomers by rhesus monkeys. Neuropsychopharmacology 2004;29:1270–81.
Green AR, Mechan AO, Elliott JM, O’Shea E, Colado MI. The pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”). Pharmacol Rev 2003;55(3):463–508.
De Win MM, Reneman L, Reitsma JB, Den Heeten GJ, Booij J, Van Den Brink W. Mood disorders and serotonin transporter density in ecstasy users—the influence of long-term abstention, dose, and gender. Psychopharmacology (Berl) 2004;173:376–82.
Xie T, Tong L, McLane MW, Hatzidimitriou G, Yuan J, McCann U, et al. Loss of serotonin transporter protein after MDMA and other ring-substituted amphetamines. Neuropsychopharmacology 2006 Jan 25; [Epub ahead of print].
Li AA, Marek GJ, Vosmer G, Seiden LS. Long-term central 5-HT depletions resulting from repeated administration of MDMA enhances the effects of single administration of MDMA on schedule-controlled behavior of rats. Pharmacol Biochem Behav 1989;33(3):641–8.
Wilkerson G, London ED. Effects of methylenedioxymethamphetamine on local cerebral glucose utilization in the rat. Neuropharmacology 1989;28(10):1129–38.
Frederick DL, Paule MG. Effects of MDMA on complex brain function in laboratory animals. Neurosci Biobehav Rev 1997;21(1):67–78.
Gamma A, Buck A, Berthold T, Liechti ME, Vollenweider FX. 3,4-Methylenedioxymethamphetamine (MDMA) modulates cortical and limbic brain activity as measured by [H2 15O]-PET in healthy humans. Neuropsychopharmacology 2000;23(4):388–95.
Holland J. Positron emission tomography findings in heavy users of MDMA. Lancet 1999;353(9152):592–3.
Hurley RA, Reneman L, Taber KH. Ecstasy in the brain: a model for neuroimaging. J Neuropsychiatry Clin Neurosci 2002;14(2):125–9.
Jarkas N, McConathy J, Voll RJ, Goodman MM. Synthesis, in vitro characterization, and radiolabeling of N,N-dimethyl-2-(2′-amino-4′-substituted-phenylthio)benzylamines: potential candidates as selective serotonin transporter radioligands. J Med Chem 2005;48(13):4254–65.
Oya S, Choi SR, Coenen H, Kung HF. New PET imaging agent for the serotonin transporter: [18F]ACF (2-[(2-amino-4-chloro-5-fluorophenyl)thio]N,N-dimethyl-benzenmethanamine). J Med Chem 2002;45(21):4716–23.
Buchert, R, Obrocki J, Thomasius R, Vaterlein O, Petersen K, Jenicke L, et al. Long-term effects of ‘ecstasy’ abuse on the human brain studied by FDG PET. Nucl Med Commun 2001;22(8):889–97.
O’Shea E, Granados R, Esteban B, Colado MI, Green AR. The relationship between the degree of neurodegeneration of rat brain 5-HT nerve terminals and the dose and frequency of administration of MDMA (‘ecstasy’). Neuropharmacology 1998;37(7):919–26.
Soto-Montenegro M, Vaquero J, Molins A, Reig S, Desco M. FDG-PET studies of the effect of MDMA in rat brain. In: 10th Annual Meeting of the Organization for Human Brain Mapping. Hungary: Budapest; 2004.
Siegel S, Vaquero JJ, Aloj L, Seidel J, Gandler WR, Green MV. Initial results from a PET/planar small animal imaging system. IEEE Trans Nucl Sci 1999;46(3):571–575.
López Herráiz J, España S, Udías JM, Vaquero JJ, Seidel J, Desco M. Full 3D-OSEM reconstruction with compressed response of the system. Book of Abstracts of 2004 IEEE Nuclear Science Symposium and Medical Imaging Conference 2004:163.
Jagoda EM, Vaquero JJ, Seidel J, Green MV, Eckelman WC. Experiment assessment of mass effects in the rat: implications for small animal PET imaging. Nucl Med Biol 2004;31(6):771–9.
Obrocki J, Buchert R, Vaterlein O, Thomasius R, Beyer W, Schiemann T. Ecstasy—long-term effects on the human central nervous system revealed by positron emission tomography. Br J Psychiatry 1999;175:186–8.
Paxinos G, Watson C. The rat brain in stereotaxic coordinates, 4th ed. San Diego, CA: Academic Press; 1998; p 474.
Colado MI, Williams JL, Green AR. The hyperthermic and neurotoxic effects of ‘Ecstasy’ (MDMA) and 3,4 methylenedioxyamphetamine (MDA) in the Dark Agouti (DA) rat, a model of the CYP2D6 poor metabolizer phenotype. Br J Pharmacol 1995;115(7):1281–9.
Malpass A, White JM, Irvine RJ, Somogyi AA, Bochner F. Acute toxicity of 3,4-methylenedioxymethamphetamine (MDMA) in Sprague-Dawley and Dark Agouti rats. Pharmacol Biochem Behav 1999;64(1):29–34.
Vincent-Viry M, Deshayes S, Mothe O, Siest G, Galteau MM. Hydroxylation of debrisoquine using perfused liver isolated from Sprague Dawley and DA rats: comparison with in-vivo results. J Pharm Pharmacol 1988;40(10):695–700.
Boobis AR, Seddon CE, Davies DS. Bufuralol 1′-hydroxylase activity of the rat. Strain differences and the effects of inhibitors. Biochem Pharmacol 1986;35(17):2961–5.
Tucker GT, Lennard MS, Ellis SW, Woods HF, Cho AK, Lin LY, et al. The demethylenation of methylenedioxymethamphetamine (“ecstasy”) by debrisoquine hydroxylase (CYP2D6). Biochem Pharmacol 1994;47(7):1151–6.
McCann UD, Slate SO, Ricaurte GA. Adverse reactions with 3,4-methylenedioxymethamphetamine (MDMA; ‘ecstasy’). Drug Saf 1996;15(2):107–15.
Parrott AC. Human psychopharmacology of Ecstasy (MDMA): a review of 15 years of empirical research. Hum Psychopharmacol 2001;16(8):557–77.
Malberg JE, Seiden LS. Small changes in ambient temperature cause large changes in 3,4-methylenedioxymethamphetamine (MDMA)-induced serotonin neurotoxicity and core body temperature in the rat. J Neurosci 1998;18(13):5086–94.
Nash JF, Roth BL, Brodkin JD, Nichols DE, Gudelsky GA. Effect of the R(−) and S(+) isomers of MDA and MDMA on phosphatidyl inositol turnover in cultured cells expressing 5-HT2A or 5-HT2C receptors. Neurosci Lett 1994;177(1–2):111–5.
de la Torre R, Farre M, Roset PN, Pizarro N, Abanades S, Segura M, et al. Human pharmacology of MDMA: pharmacokinetics, metabolism, and disposition. Ther Drug Monit 2004;26(2):137–44.
Henry JA, Jeffreys KJ, Dawling S. Toxicity and deaths from 3,4-methylenedioxymethamphetamine (“ecstasy”). Lancet 1992;340(8816):384–7.
Heffron JJ. Malignant hyperthermia: biochemical aspects of the acute episode. Br J Anaesth 1988;60(3):274–8.
Hall GM, Lucke JN, Lovell R, Lister D. Porcine malignant hyperthermia. VII: hepatic metabolism. Br J Anaesth 1980;52(1):11–7.
Andreu V, Mas A, Bruguera M, Salmeron JM, Moreno V, Nogue S, et al. Ecstasy: a common cause of severe acute hepatotoxicity. J Hepatol 1998;29(3):394–7.
Montgomery H, Myerson S. 3,4-methylenedioxymethamphetamine (MDMA, or “ecstasy”) and associated hypoglycemia. Am J Emerg Med 1997;15(2):218.
Lange-Brock N, Berg T, Muller AR, Fliege H, Neuhaus P, Wiedenmann B, et al. Acute liver failure following the use of ecstasy (MDMA). Z Gastroenterol 2002;40(8):581–6.
Vollenweider FX, Liechti ME, Gamma A, Greer G, Geyer M. Acute psychological and neurophysiological effects of MDMA in humans. J Psychoact Drugs 2002;34(2):171–84.
McCulloch J, Savaki HE, Jehle J, Sokoloff L. Local cerebral glucose utilization in hypothermic and hyperthermic rats. J Neurochem 1982;39(1):255–8.
Nunneley SA, Martin CC, Slauson JW, Hearon CM, Nickerson LD, Mason PA. Changes in regional cerebral metabolism during systemic hyperthermia in humans. J Appl Physiol 2002;92(2):846–51.
Ricaurte GA, McCann UD. Recognition and management of complications of new recreational drug use. Lancet 2005;365(9477):2137–45.
Carlsson M, Svensson K, Eriksson E, Carlsson A. Rat brain serotonin: biochemical and functional evidence for a sex difference. J Neural Transm 1985;63(3–4):297–313.
Palenicek T, Votava M, Bubenikova V, Horacek J. Increased sensitivity to the acute effects of MDMA (“ecstasy”) in female rats. Physiol Behav 2005;86(4):546–53.
Acknowledgements
This work has been supported by grants from “Red Temática G03/185 and G03/032: Fondo de Investigación Sanitaria”, “Agencia Antidroga de la Comunidad de Madrid” and “Ministerio del Interior”. We thank the National Institute on Drug Abuse for supplying the MDMA and the Atomic, Molecular, and Nuclear Physics Department, Universidad Complutense de Madrid, for reconstructing the PET images.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Soto-Montenegro, M.L., Vaquero, J.J., Arango, C. et al. Effects of MDMA on blood glucose levels and brain glucose metabolism. Eur J Nucl Med Mol Imaging 34, 916–925 (2007). https://doi.org/10.1007/s00259-006-0262-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-006-0262-8